Abnormal spermatogenesis and male infertility in testicular zinc finger protein Zfp318-knockout mice
- PMID: 27385512
- PMCID: PMC11520953
- DOI: 10.1111/dgd.12301
Abnormal spermatogenesis and male infertility in testicular zinc finger protein Zfp318-knockout mice
Abstract
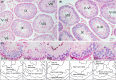
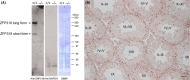
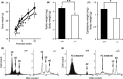
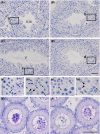
Zfp318, a mouse gene with a Cys2/His2 zinc finger motif, is mainly expressed in germ cells in the testis. It encodes two alternative transcripts, which regulate androgen receptor-mediated transcriptional activation or repression by overexpression of them. However, the role of Zfp318 is still obscure in vivo, especially in spermatogenesis. To elucidate the role of Zfp318 during gamete production, we established a knockout mouse line. Zfp318-null male mice exhibited infertility, whereas Zfp318-null female mice displayed normal fertility. ZFP318 was expressed during multiple stages of spermatogenesis, from spermatocytes to round spermatids. The nuclei of secondary spermatocytes showed high levels of expression. Histological analysis and quantitative analysis of DNA content showed decreased numbers of both spermatids in the seminiferous tubules and mature spermatozoa in the epididymides of Zfp318-null mice. These results suggest that Zfp318 is expressed as a functional protein in testicular germ cells and plays an important role in meiosis during spermatogenesis.
Keywords: male infertility; sperm count; spermatogenesis; testis; zinc finger.
© 2016 The Authors Development, Growth & Differentiation published by John Wiley & Sons Australia, Ltd on behalf of Japanese Society of Developmental Biologists.
Figures

Similar articles
-
Dicer1 depletion in male germ cells leads to infertility due to cumulative meiotic and spermiogenic defects.PLoS One. 2011;6(10):e25241. doi: 10.1371/journal.pone.0025241. Epub 2011 Oct 5. PLoS One. 2011. PMID: 21998645 Free PMC article.
-
Deletion of Hnrnpk Gene Causes Infertility in Male Mice by Disrupting Spermatogenesis.Cells. 2022 Apr 9;11(8):1277. doi: 10.3390/cells11081277. Cells. 2022. PMID: 35455958 Free PMC article.
-
Dicer is required for haploid male germ cell differentiation in mice.PLoS One. 2011;6(9):e24821. doi: 10.1371/journal.pone.0024821. Epub 2011 Sep 16. PLoS One. 2011. PMID: 21949761 Free PMC article.
-
Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice.Endocr Rev. 2009 Apr;30(2):119-32. doi: 10.1210/er.2008-0025. Epub 2009 Jan 27. Endocr Rev. 2009. PMID: 19176467 Free PMC article. Review.
-
Androgen receptor roles in spermatogenesis and infertility.Best Pract Res Clin Endocrinol Metab. 2015 Aug;29(4):595-605. doi: 10.1016/j.beem.2015.04.006. Epub 2015 Apr 25. Best Pract Res Clin Endocrinol Metab. 2015. PMID: 26303086 Review.
Cited by
-
Oxidative Stress and Male Infertility: The Protective Role of Antioxidants.Medicina (Kaunas). 2023 Oct 4;59(10):1769. doi: 10.3390/medicina59101769. Medicina (Kaunas). 2023. PMID: 37893487 Free PMC article. Review.
-
Zinc and selenium attenuate quaternary heavy metal mixture-induced testicular damage via amplification of the antioxidant system, reduction in metal accumulation, inflammatory and apoptotic biomarkers.Toxicol Res. 2023 May 18;39(3):497-515. doi: 10.1007/s43188-023-00187-z. eCollection 2023 Jul. Toxicol Res. 2023. PMID: 37398573 Free PMC article.
-
Comparative effects of combined use of alcohol with cannabis and tobacco on testicular function in rats.Toxicol Res (Camb). 2021 Jun 29;10(4):761-770. doi: 10.1093/toxres/tfab060. eCollection 2021 Aug. Toxicol Res (Camb). 2021. PMID: 34484667 Free PMC article.
-
Germline-dependent transmission of male reproductive traits induced by an endocrine disruptor, di-2-ethylhexyl phthalate, in future generations.Sci Rep. 2020 Mar 31;10(1):5705. doi: 10.1038/s41598-020-62584-w. Sci Rep. 2020. PMID: 32235866 Free PMC article.
-
Genetic profiling of azoospermic men to identify the etiology and predict reproductive potential.J Assist Reprod Genet. 2024 Apr;41(4):1111-1124. doi: 10.1007/s10815-024-03045-5. Epub 2024 Feb 26. J Assist Reprod Genet. 2024. PMID: 38403804 Free PMC article.
References
-
- Ballow, D. , Meistrich, M. L. , Matzuk, M. & Rajkovic, A. 2006. Sohlh1 is essential for spermatogonial differentiation. Dev. Biol. 294, 161–167. - PubMed
-
- Blendy, J. A. , Kaestner, K. H. , Weinbauer, G. F. , Nieschlag, E. & Schutz, G. 1996. Severe impairment of spermatogenesis in mice lacking the CREM gene. Nature 380, 162–165. - PubMed
-
- Buaas, F. W. , Kirsh, A. L. , Sharma, M. , Mclean, D. J. , Morris, J. L. , Griswold, M. D. , De Rooij, D. G. & Braun, R. E. 2004. Plzf is required in adult male germ cells for stem cell self‐renewal. Nat. Genet. 36, 647–652. - PubMed
-
- Chizaki, R. , Yao, I. , Katano, T. , Matsuda, T. & Ito, S. 2012. Restricted expression of Ovol2/MOVO in XY body of mouse spermatocytes at the pachytene stage. J. Androl. 33, 277–286. - PubMed
-
- Costoya, J. A. , Hobbs, R. M. , Barna, M. , Cattoretti, G. , Manova, K. , Sukhwani, M. , Orwig, K. E. , Wolgemuth, D. J. & Pandolfi, P. P. 2004. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat. Genet. 36, 653–659. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

