Fatty acid metabolism and the basis of brown adipose tissue function
- PMID: 27386151
- PMCID: PMC4916887
- DOI: 10.1080/21623945.2015.1122857
Fatty acid metabolism and the basis of brown adipose tissue function
Abstract
Obesity has reached epidemic proportions, leading to severe associated pathologies such as insulin resistance, cardiovascular disease, cancer and type 2 diabetes. Adipose tissue has become crucial due to its involvement in the pathogenesis of obesity-induced insulin resistance, and traditionally white adipose tissue has captured the most attention. However in the last decade the presence and activity of heat-generating brown adipose tissue (BAT) in adult humans has been rediscovered. BAT decreases with age and in obese and diabetic patients. It has thus attracted strong scientific interest, and any strategy to increase its mass or activity might lead to new therapeutic approaches to obesity and associated metabolic diseases. In this review we highlight the mechanisms of fatty acid uptake, trafficking and oxidation in brown fat thermogenesis. We focus on BAT's morphological and functional characteristics and fatty acid synthesis, storage, oxidation and use as a source of energy.
Keywords: brown adipose tissue; fatty acid oxidation; lipid metabolism; obesity.
Figures

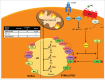
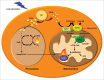
References
-
- World Health Organization Obesity and overweight. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed 1October2015)
-
- Mathis D. Immunological goings-on in visceral adipose tissue. Cell Metab 2013; 17:851-9; PMID:23747244; http://dx.doi.org/ 10.1016/j.cmet.2013.05.008 - DOI - PMC - PubMed
-
- Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell 2012; 148:852-71; PMID:22385956; http://dx.doi.org/ 10.1016/j.cell.2012.02.017 - DOI - PMC - PubMed
-
- Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology 2007; 132:2169-80; PMID:17498510; http://dx.doi.org/ 10.1053/j.gastro.2007.03.059 - DOI - PubMed
-
- Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010; 140:900-17; PMID:20303879; http://dx.doi.org/ 10.1016/j.cell.2010.02.034 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
