Biodegradation of different formulations of polyhydroxybutyrate films in soil
- PMID: 27386248
- PMCID: PMC4912537
- DOI: 10.1186/s40064-016-2480-2
Biodegradation of different formulations of polyhydroxybutyrate films in soil
Abstract
Background: Petroleum polymers contribute to non-degradable waste materials and it would therefore be desirable to produce ecofriendly degradable materials. Biodegradation of polyhydroxybutyrate (PHB) in the presence of oligomer hydrolase and PHB depolymerase gave 3-hydroxybutyric acid which could be oxidized to acetyl acetate. Several bacteria and fungi can degrade PHB in the soil.
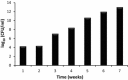
Results: Biodegradation of PHB showed a significant decrease in the molecular weight (Mw), number-average molecular weight (Mn) and the dispersity (Mw/Mn) for all the film formulations. Nanofibers of PHB and its composites showed faster degradation compared to other films and displayed complete degradation after 3 weeks. The SEM micrographs showed various surface morphology changes including alterations in appearance of pores, cavity, grooves, incisions, slots and pointers. Such changes were due to the growth of microorganisms that secreted PHB depolymerase enzyme which lead to the biopolymer films degradation. However, PHB nanofibers and its composites films in the presence of TiO2 demonstrated more surface changes with rupture of most nanofibers in which there was a drop in fibres diameter.
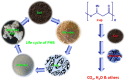
Conclusions: The degradation of biopolymers help to overcome some of the pollution problems associated with the use of petroleum polymers. PHB nanofiber and its TiO2 composite were degraded faster compared to other PHB film types due to their three dimensional and high surface area structures. The presence of TiO2 nanoparticles in the composite films slowdown the degradation process compared to PHB films. Additionally, the PHB and its composite films that were prepared from UV treated PHB films led to acceleration of the degradation.Graphical abstractBiodegradation of polyhydroxybutyrate films in soil.
Keywords: Biodegradation; Electrospinning; Nanofibers; Polyhydroxyalkanoates; Polyhydroxybutyrate; UV treatment.
Figures






References
-
- Ahmad R, Sardar M. TiO2 nanoparticles as an antibacterial agents against E. coli. Int J Innov Res Sci Eng Technol. 2013;2:3569–3574.
-
- Al-Rawi KM, Khalaf Allah AM. Agriculture experiments design and analysis. 2. Mosul: Mosul University Press; 2000.
-
- Altaee N, Fahdil A, Yousif E, Sudesh K. Recovery and subsequent characterization of polyhydroxybutyrate from Rhodococcus equi cells grown on crude palm kernel oil. J Taibah Univ Sci. 2015
-
- Avella M, De Vlieger JJ, Errico ME, Fischer S, Vacca P, Volpe MG. Biodegradable starch/clay nanocomposite films for food packaging applications. Food Chem. 2005;93:467–474. doi: 10.1016/j.foodchem.2004.10.024. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

