Experimental Phage Therapy for Burkholderia pseudomallei Infection
- PMID: 27387381
- PMCID: PMC4936672
- DOI: 10.1371/journal.pone.0158213
Experimental Phage Therapy for Burkholderia pseudomallei Infection
Abstract
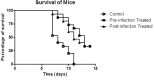
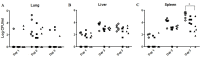
Burkholderia pseudomallei is an intracellular Gram-negative bacterial pathogen intrinsically resistant to a variety of antibiotics. Phages have been developed for use as an alternative treatment therapy, particularly for bacterial infections that do not respond to conventional antibiotics. In this study, we investigated the use of phages to treat cells infected with B. pseudomallei. Phage C34 isolated from seawater was purified and characterised on the basis of its host range and morphology using transmission electron microscopy (TEM). Phage C34 was able to lyse 39.5% of B. pseudomallei clinical strains. Due to the presence of contractile tail, phage C34 is classified as a member of the family Myoviridae, a tailed double-stranded DNA virus. When 2 × 105 A549 cells were exposed to 2 × 107 PFU of phage C34, 24 hours prior to infection with 2 × 106 CFU of B. pseudomallei, it was found that the survivability of the cells increased to 41.6 ± 6.8% as compared to 22.8 ± 6.0% in untreated control. Additionally, application of phage successfully rescued 33.3% of mice infected with B. pseudomallei and significantly reduced the bacterial load in the spleen of the phage-treated mice. These findings indicate that phage can be a potential antimicrobial agent for B. pseudomallei infections.
Conflict of interest statement
Figures

References
-
- Puthucheary SD. Melioidosis in Malaysia. Med J Malaysia. 2009;64(4):266–74. Epub 2010/10/20. . - PubMed
-
- Sookpranee M, Boonma P, Susaengrat W, Bhuripanyo K, Punyagupta S. Multicenter prospective randomized trial comparing ceftazidime plus co-trimoxazole with chloramphenicol plus doxycycline and co-trimoxazole for treatment of severe melioidosis. Antimicrob Agents Chemother. 1992;36(1):158–62. Epub 1992/01/01. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

