Ionizing Radiation-Induced Endothelial Cell Senescence and Cardiovascular Diseases
- PMID: 27387862
- PMCID: PMC4997805
- DOI: 10.1667/RR14445.1
Ionizing Radiation-Induced Endothelial Cell Senescence and Cardiovascular Diseases
Abstract
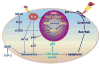
Exposure to ionizing radiation induces not only apoptosis but also senescence. While the role of endothelial cell apoptosis in mediating radiation-induced acute tissue injury has been extensively studied, little is known about the role of endothelial cell senescence in the pathogenesis of radiation-induced late effects. Senescent endothelial cells exhibit decreased production of nitric oxide and expression of thrombomodulin, increased expression of adhesion molecules, elevated production of reactive oxygen species and inflammatory cytokines and an inability to proliferate and form capillary-like structures in vitro. These findings suggest that endothelial cell senescence can lead to endothelial dysfunction by dysregulation of vasodilation and hemostasis, induction of oxidative stress and inflammation and inhibition of angiogenesis, which can potentially contribute to radiation-induced late effects such as cardiovascular diseases (CVDs). In this article, we discuss the mechanisms by which radiation induces endothelial cell senescence, the roles of endothelial cell senescence in radiation-induced CVDs and potential strategies to prevent, mitigate and treat radiation-induced CVDs by targeting senescent endothelial cells.
Figures



References
-
- Adams MJ, Lipsitz SR, Colan SD, Tarbell NJ, Treves ST, Diller L, et al. Cardiovascular status in long-term survivors of Hodgkin’s disease treated with chest radiotherapy. J Clin Oncol. 2004;22:3139–48. - PubMed
-
- Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557–65. - PubMed
-
- Early Breast Cancer Trialists Collaborative Group. Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Lancet. 2000;355:1757–70. - PubMed
-
- Heidenreich PA, Kapoor JR. Radiation induced heart disease: systemic disorders in heart disease. Heart. 2009;95:252–8. - PubMed
-
- Martinou M, Gaya A. Cardiac complications after radical radiotherapy. Semin Oncol. 2013;40:178–85. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

