Diabetes Mellitus Impairs Cognitive Function in Middle-Aged Rats and Neurological Recovery in Middle-Aged Rats After Stroke
- PMID: 27387991
- PMCID: PMC4961558
- DOI: 10.1161/STROKEAHA.115.012578
Diabetes Mellitus Impairs Cognitive Function in Middle-Aged Rats and Neurological Recovery in Middle-Aged Rats After Stroke
Abstract
Background and purpose: Diabetes mellitus (DM) is a common metabolic disease among the middle-aged and older population, which leads to an increase of stroke incidence and poor stroke recovery. The present study was designed to investigate the impact of DM on brain damage and on ischemic brain repair after stroke in aging animals.
Methods: DM was induced in middle-aged rats (13 months) by administration of nicotinamide and streptozotocin. Rats with confirmed hyperglycemia status 30 days after nicotinamide-streptozotocin injection and age-matched non-DM rats were subjected to embolic middle cerebral artery occlusion.
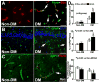
Results: Middle-aged rats subjected to nicotinamide-streptozotocin injection became hyperglycemic and developed cognitive deficits 2 months after induction of DM. Histopathologic analysis revealed that there was sporadic vascular disruption, including cerebral microvascular thrombosis, blood-brain barrier leakage, and loss of paravascular aquaporin-4 in the hippocampi. Importantly, middle-aged DM rats subjected to stroke had exacerbated sensorimotor and cognitive deficits compared with age-matched non-DM ischemic rats during stroke recovery. Compared with age-matched non-DM ischemic rats, DM ischemic rats exhibited aggravated neurovascular disruption in the bilateral hippocampi and white matter, suppressed stroke-induced neurogenesis and oligodendrogenesis, and impaired dendritic/spine plasticity. However, DM did not enlarge infarct volume.
Conclusions: Our data suggest that DM exacerbates neurovascular damage and hinders brain repair processes, which likely contribute to the impairment of stroke recovery.
Keywords: blood–brain barrier; diabetes mellitus; hyperglycemia; recovery; stroke.
© 2016 American Heart Association, Inc.
Figures





References
-
- Lukovits TG, Mazzone TM, Gorelick TM. Diabetes mellitus and cerebrovascular disease. Neuroepidemiology. 1999;18:1–14. - PubMed
-
- Jorgensen H, Nakayama H, Raaschou HO, Olsen TS. Stroke in patients with diabetes. The Copenhagen stroke study. Stroke. 1994;25:1977–1984. - PubMed
-
- Newman GC, Bang H, Hussain SI, Toole JF. Association of diabetes, homocysteine, and hdl with cognition and disability after stroke. Neurology. 2007;69:2054–2062. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

