Tumor-suppressive effects of atelocollagen-conjugated hsa-miR-520d-5p on un-differentiated cancer cells in a mouse xenograft model
- PMID: 27388711
- PMCID: PMC4936056
- DOI: 10.1186/s12885-016-2467-y
Tumor-suppressive effects of atelocollagen-conjugated hsa-miR-520d-5p on un-differentiated cancer cells in a mouse xenograft model
Erratum in
-
Correction to: Tumor-suppressive effects of atelocollagen-conjugated hsa-miR-520d-5p on un-differentiated cancer cells in a mouse xenograft model.BMC Cancer. 2017 Oct 2;17(1):666. doi: 10.1186/s12885-017-3653-2. BMC Cancer. 2017. PMID: 28969618 Free PMC article. No abstract available.
Abstract
Background: We previously demonstrated that hsa-miR-520d-5p can convert cancer cells into induced pluripotent stem cells (iPSCs) or mesenchymal stem cells (MSCs) via a demethylation process and p53 upregulation in vivo. Additionally, we have reported the non-tumorigenic effect of miR-520d-5p on normal human cells, including fibroblasts.
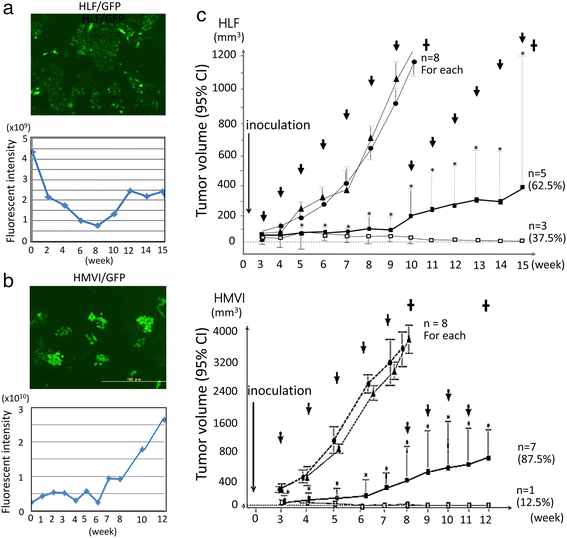
Methods: We used atelocollagen-conjugated miR-520d-5p (520d/atelocollagen) to confirm the possibility of a therapeutic effect on cancer cells. We traced the size and signal intensity of GFP-expressing tumors in mice each week, beginning 4 weeks after subcutaneous inoculation.
Results: 520d/atelocollagen treatment suppressed tumor growth by greater than 80 % each week relative to controls and resulted in an approximately 30 % disappearance of tumors. In mice whose tumors disappeared, the existence of human genomic material at the injection site was examined by quantitative Alu-PCR, and we confirmed the co-existence of both species-derived cells. In every site where a tumor disappeared in immunodeficient mice, GFP protein was expressed in the connective tissues, and approximately 0.1 % of the extracted DNA contained human genomic material. We could not identify any adverse effects in vivo.
Conclusions: This is the first report to confirm an inhibitory effect of 520d/atelocollagen on cancer cells in vivo. The development of optimized modifications of this carrier is expected to enhance the efficiency of entry into tumor cells and the induction of its inhibitory effect.
Keywords: Atelocollagen; Cancer; Therapeutic effect; Xenograft model; miR-520d-5p.
Figures





Similar articles
-
miR-520d-5p can reduce the mutations in hepatoma cancer cells and iPSCs-derivatives.BMC Cancer. 2019 Jun 15;19(1):587. doi: 10.1186/s12885-019-5786-y. BMC Cancer. 2019. PMID: 31202279 Free PMC article.
-
SIRP Alpha Protein Downregulates in Human Astrocytoma: Presumptive Involvement of Hsa-miR-520d-5p and Hsa-miR-520d-3p.Mol Neurobiol. 2017 Dec;54(10):8162-8169. doi: 10.1007/s12035-016-0302-8. Epub 2016 Nov 29. Mol Neurobiol. 2017. PMID: 27900675
-
Correction to: Tumor-suppressive effects of atelocollagen-conjugated hsa-miR-520d-5p on un-differentiated cancer cells in a mouse xenograft model.BMC Cancer. 2017 Oct 2;17(1):666. doi: 10.1186/s12885-017-3653-2. BMC Cancer. 2017. PMID: 28969618 Free PMC article. No abstract available.
-
Hsa-miR-520d converts fibroblasts into CD105+ populations.Drugs R D. 2014 Dec;14(4):253-64. doi: 10.1007/s40268-014-0064-6. Drugs R D. 2014. PMID: 25303886 Free PMC article.
-
Atomoxetine suppresses radioresistance in glioblastoma via circATIC/miR-520d-5p/Notch2-Hey1 axis.Cell Commun Signal. 2024 Nov 5;22(1):532. doi: 10.1186/s12964-024-01915-0. Cell Commun Signal. 2024. PMID: 39501373 Free PMC article.
Cited by
-
miR-520d-5p can reduce the mutations in hepatoma cancer cells and iPSCs-derivatives.BMC Cancer. 2019 Jun 15;19(1):587. doi: 10.1186/s12885-019-5786-y. BMC Cancer. 2019. PMID: 31202279 Free PMC article.
-
SIRP Alpha Protein Downregulates in Human Astrocytoma: Presumptive Involvement of Hsa-miR-520d-5p and Hsa-miR-520d-3p.Mol Neurobiol. 2017 Dec;54(10):8162-8169. doi: 10.1007/s12035-016-0302-8. Epub 2016 Nov 29. Mol Neurobiol. 2017. PMID: 27900675
-
Role of non-coding RNA in pancreatic cancer.Oncol Lett. 2019 Oct;18(4):3963-3973. doi: 10.3892/ol.2019.10758. Epub 2019 Aug 16. Oncol Lett. 2019. PMID: 31579086 Free PMC article. Review.
-
Correction to: Tumor-suppressive effects of atelocollagen-conjugated hsa-miR-520d-5p on un-differentiated cancer cells in a mouse xenograft model.BMC Cancer. 2017 Oct 2;17(1):666. doi: 10.1186/s12885-017-3653-2. BMC Cancer. 2017. PMID: 28969618 Free PMC article. No abstract available.
-
Current Achievements and Applications of Transcriptomics in Personalized Cancer Medicine.Int J Mol Sci. 2021 Jan 31;22(3):1422. doi: 10.3390/ijms22031422. Int J Mol Sci. 2021. PMID: 33572595 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

