Insights into blood cell formation from hemogenic endothelium in lesser-known anatomic sites
- PMID: 27389484
- PMCID: PMC5801748
- DOI: 10.1002/dvdy.24430
Insights into blood cell formation from hemogenic endothelium in lesser-known anatomic sites
Abstract
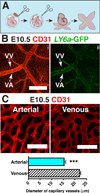
Background: Hematopoietic stem and progenitor cells (HSPCs) are generated de novo in the embryo in a process termed the endothelial to hematopoietic transition (EHT). EHT is most extensively studied in the yolk sac and dorsal aorta. Recently new sites of hematopoiesis have been described, including the heart, somites, head, and venous plexus of the yolk sac.
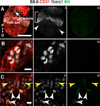
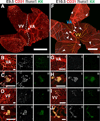
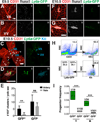
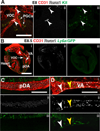
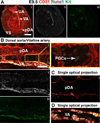
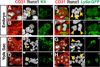
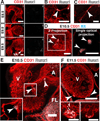
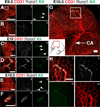
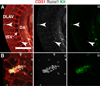
Results: We examined sites of HSPC formation in well-studied and in less well-known sites by mapping the expression of the key EHT factor Runx1 along with several other markers by means of confocal microscopy. We identified sites of HSPC formation in the head, heart and somites. We also identified sites of HSPC formation in both the arterial and venous plexuses of the yolk sac, and show that progenitors with lymphoid potential are enriched in hematopoietic clusters in close proximity to arteries. Furthermore, we demonstrate that many of the cells in hematopoietic clusters resemble monocytes or granulocytes based on nuclear shape.
Conclusions: We identified sites of HSPC formation in the head, heart, and somites, confirming that embryonic hematopoiesis is less spatially restricted than previously thought. Furthermore, we show that HSPCs in the yolk sac with lymphoid potential are located in closer proximity to arteries than to veins. Developmental Dynamics 245:1011-1028, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: Ly6a; Runx1; embryo; head; heart; hematopoiesis; nuclear shape; somites; yolk sac.
© 2016 Wiley Periodicals, Inc.
Figures

References
-
- Biermann H, Pietz B, Dreier R, Schmid KW, Sorg C, Sunderkötter C. Murine leukocytes with ring-shaped nuclei include granulocytes, monocytes, and their precursors. J Leukoc Biol. 1999;65:217–231. - PubMed
-
- Bigas A, Guiu J, Gama-Norton L. Notch and Wnt signaling in the emergence of hematopoietic stem cells. Blood Cells Mol Dis. 2013;51:264–270. - PubMed
-
- Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–120. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

