Metarhizium brunneum Blastospore Pathogenesis in Aedes aegypti Larvae: Attack on Several Fronts Accelerates Mortality
- PMID: 27389584
- PMCID: PMC4936676
- DOI: 10.1371/journal.ppat.1005715
Metarhizium brunneum Blastospore Pathogenesis in Aedes aegypti Larvae: Attack on Several Fronts Accelerates Mortality
Abstract
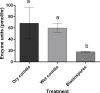
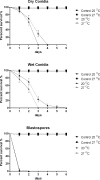
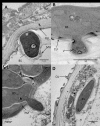
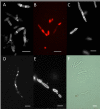
Aedes aegypti is the vector of a wide range of diseases (e.g. yellow fever, dengue, Chikungunya and Zika) which impact on over half the world's population. Entomopathogenic fungi such as Metarhizium anisopliae and Beauveria bassiana have been found to be highly efficacious in killing mosquito larvae but only now are the underlying mechanisms for pathogenesis being elucidated. Recently it was shown that conidia of M. anisopliae caused stress induced mortality in Ae. aegypti larvae, a different mode of pathogenicity to that normally seen in terrestrial hosts. Blastospores constitute a different form of inoculum produced by this fungus when cultured in liquid media and although blastospores are generally considered to be more virulent than conidia no evidence has been presented to explain why. In our study, using a range of biochemical, molecular and microscopy methods, the infection process of Metarhizium brunneum (formerly M. anisopliae) ARSEF 4556 blastospores was investigated. It appears that the blastospores, unlike conidia, readily adhere to and penetrate mosquito larval cuticle. The blastospores are readily ingested by the larvae but unlike the conidia are able infect the insect through the gut and rapidly invade the haemocoel. The fact that pathogenicity related genes were upregulated in blastospores exposed to larvae prior to invasion, suggests the fungus was detecting host derived cues. Similarly, immune and defence genes were upregulated in the host prior to infection suggesting mosquitoes were also able to detect pathogen-derived cues. The hydrophilic blastospores produce copious mucilage, which probably facilitates adhesion to the host but do not appear to depend on production of Pr1, a cuticle degrading subtilisin protease, for penetration since protease inhibitors did not significantly alter blastospore virulence. The fact the blastospores have multiple routes of entry (cuticle and gut) may explain why this form of the inoculum killed Ae. aegypti larvae in a relatively short time (12-24hrs), significantly quicker than when larvae were exposed to conidia. This study shows that selecting the appropriate form of inoculum is important for efficacious control of disease vectors such as Ae. aegypti.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- WHO. Yellow fever fact sheet N°100. Weekly Epidemiological Record. 2010;85:33–6. - PubMed
-
- WHO. Global strategy for dengue prevention and control 2012–2020: World Health Organization; 2012.
-
- Marcondes CB, Ximenes MdFFd. Zika virus in Brazil and the danger of infestation by Aedes (Stegomyia) mosquitoes. Revista da Sociedade Brasileira de Medicina Tropical. 2015;(AHEAD):0-. - PubMed
-
- WHO. Epidemiological update on Zika virus infection—16 October 2015 2015 [cited 2015 14-12-2015]. Available from: http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=....
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

