Dose-response efficacy and safety of PA21 in Japanese hemodialysis patients with hyperphosphatemia: a randomized, placebo-controlled, double-blind, Phase II study
- PMID: 27389681
- PMCID: PMC5486467
- DOI: 10.1007/s10157-016-1299-z
Dose-response efficacy and safety of PA21 in Japanese hemodialysis patients with hyperphosphatemia: a randomized, placebo-controlled, double-blind, Phase II study
Erratum in
-
Erratum to: Dose-response efficacy and safety of PA21 in Japanese hemodialysis patients with hyperphosphatemia: a randomized, placebo-controlled, double-blind, Phase II study.Clin Exp Nephrol. 2017 Jun;21(3):523. doi: 10.1007/s10157-016-1350-0. Clin Exp Nephrol. 2017. PMID: 27832343 Free PMC article. No abstract available.
Abstract
Background: Hyperphosphatemia is common in chronic kidney disease (CKD) and associated with mortality and morbidity. We aimed to evaluate the dose-dependent efficacy and safety of PA21 (sucroferric oxyhydroxide), an iron-based phosphate binder, in Japanese hemodialysis patients with hyperphosphatemia.
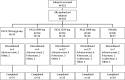
Methods: In this double-blind, multicenter, Phase II study, 183 patients were randomized to placebo or PA21 at doses of 250, 500, 750, or 1000 mg (based on iron content) three times/day for 6 weeks. The primary efficacy endpoint was the mean change in serum phosphorus levels from baseline to end of treatment in each group. Adverse reactions were evaluated.
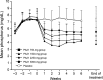
Results: The change in serum phosphorus level was significantly greater in each PA21 group than in the placebo group (analysis of covariance: P < 0.001 for all groups). A dose-dependent change in serum phosphorus levels was observed in the PA21 groups. A notable decrease in mean serum phosphorus levels to the target level of ≤6 mg/dL was shown starting at Week 1 in all PA21 groups. The cumulative achievement rates for target serum phosphorus level at the end of treatment were generally >80 % in all PA21 groups. The major adverse reaction reported was diarrhea; however, most cases were mild.
Conclusions: PA21 was an effective and safe treatment that decreased serum phosphorus levels starting at 1 week of treatment when administered as one 250-mg tablet three times/day. PA21 demonstrated a dose-dependent phosphorus lowering effect up to 3000 mg/day. PA21 may be a new treatment alternative with relatively low pill burden for Japanese hemodialysis patients with hyperphosphatemia.
Keywords: Hemodialysis; Hyperphosphatemia; Japanese; PA21 compound; Phosphate binder; Sucroferric oxyhydroxide.
Conflict of interest statement
Fumihiko Koiwa is an advisor for and has received consulting fees from Kissei Pharmaceutical Co., Ltd. Akira Terao has received consulting fees from Kissei Pharmaceutical Co., Ltd. This study was sponsored by Kissei Pharmaceutical Co., Ltd.
Figures


References
-
- National Kidney Foundation K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1–S201. - PubMed
-
- Moe S, Drüeke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G, Kidney Disease: improving Global Outcomes (KDIGO) Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–1953. doi: 10.1038/sj.ki.5000414. - DOI - PubMed
-
- Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, McAllister CJ, Budoff MJ, Salusky IB, Kopple JD. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70:771–780. doi: 10.1038/sj.ki.5001514. - DOI - PubMed
-
- Kidney Disease Improving Global Outcomes (KDIGO) CKD-MBD Work Group KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD. Kidney Int. 2009;76:S1–S130. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

