Biochemical and structural analysis of the interaction between β-amyloid and fibrinogen
- PMID: 27389717
- PMCID: PMC5000847
- DOI: 10.1182/blood-2016-03-705228
Biochemical and structural analysis of the interaction between β-amyloid and fibrinogen
Abstract
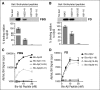
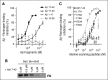
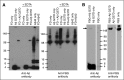
The majority of patients with Alzheimer disease (AD) suffer from impaired cerebral circulation. Accumulating evidence suggests that fibrinogen, the main protein component of blood clots, plays an important role in this circulatory dysfunction in AD. Fibrinogen interacts with β-amyloid (Aβ), forming plasmin-resistant abnormal blood clots, and increased fibrin deposition is found in the brains of AD patients and mouse models. In this study, we investigated the biochemical and structural details of the Aβ-fibrinogen interaction. We identified the central region of Aβ42 as the most critical region for the interaction, which can be inhibited by specific antibodies against the central region of Aβ and by naturally occurring p3 peptides, Aβ17-40 and Aβ17-42. X-ray crystallographic analysis revealed that Aβ42 binding to fragment D of fibrinogen induced a structural change in the C-terminal region of the fibrinogen β-chain (β384-393). Furthermore, we identified an additional Aβ-binding site within the αC region of fibrinogen. Aβ binding to this αC region blocked plasmin-mediated fibrin cleavage at this site, resulting in the generation of increased levels of a plasmin-resistant fibrin degradation fragment. Overall, our study elucidates the Aβ-fibrinogen interaction and clarifies the mechanism by which Aβ-fibrinogen binding delays fibrinolysis by plasmin. These results may facilitate the development of effective therapeutics against the Aβ-fibrinogen interaction to treat cerebrovascular abnormalities in AD.
© 2016 by The American Society of Hematology.
Figures



References
-
- Narayan PJ, Kim SL, Lill C, et al. Assessing fibrinogen extravasation into Alzheimer’s disease brain using high-content screening of brain tissue microarrays. J Neurosci Methods. 2015;247:41–49. - PubMed
-
- Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol. 2012;34(1):43–62. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

