Wars2 is a determinant of angiogenesis
- PMID: 27389904
- PMCID: PMC4941120
- DOI: 10.1038/ncomms12061
Wars2 is a determinant of angiogenesis
Abstract
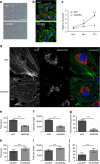
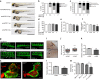
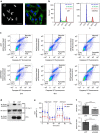
Coronary flow (CF) measured ex vivo is largely determined by capillary density that reflects angiogenic vessel formation in the heart in vivo. Here we exploit this relationship and show that CF in the rat is influenced by a locus on rat chromosome 2 that is also associated with cardiac capillary density. Mitochondrial tryptophanyl-tRNA synthetase (Wars2), encoding an L53F protein variant within the ATP-binding motif, is prioritized as the candidate at the locus by integrating genomic data sets. WARS2(L53F) has low enzyme activity and inhibition of WARS2 in endothelial cells reduces angiogenesis. In the zebrafish, inhibition of wars2 results in trunk vessel deficiencies, disordered endocardial-myocardial contact and impaired heart function. Inhibition of Wars2 in the rat causes cardiac angiogenesis defects and diminished cardiac capillary density. Our data demonstrate a pro-angiogenic function for Wars2 both within and outside the heart that may have translational relevance given the association of WARS2 with common human diseases.
Figures

References
-
- Simons M. Angiogenesis: where do we stand now? Circulation 111, 1556–1566 (2005). - PubMed
-
- Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat. Rev. Drug Discov. 6, 273–286 (2007). - PubMed
-
- Sano M. et al.. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature 446, 444–448 (2007). - PubMed
-
- Oka T., Akazawa H., Naito A. T. & Komuro I. Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ. Res. 114, 565–571 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

