Mesenchymal stem cells in regenerative rehabilitation
- PMID: 27390452
- PMCID: PMC4932093
- DOI: 10.1589/jpts.28.1943
Mesenchymal stem cells in regenerative rehabilitation
Abstract
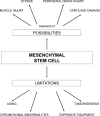
[Purpose] Regenerative medicine and rehabilitation contribute in many ways to a specific plan of care based on a patient's medical status. The intrinsic self-renewing, multipotent, regenerative, and immunosuppressive properties of mesenchymal stem cells offer great promise in the treatment of numerous autoimmune, degenerative, and graft-versus-host diseases, as well as tissue injuries. As such, mesenchymal stem cells represent a therapeutic fortune in regenerative medicine. The aim of this review is to discuss possibilities, limitations, and future clinical applications of mesenchymal stem cells. [Subjects and Methods] The authors have identified and discussed clinically and scientifically relevant articles from PubMed that have met the inclusion criteria. [Results] Direct treatment of muscle injuries, stroke, damaged peripheral nerves, and cartilage with mesenchymal stem cells has been demonstrated to be effective, with synergies seen between cellular and physical therapies. Over the past few years, several researchers, including us, have shown that there are certain limitations in the use of mesenchymal stem cells. Aging and spontaneous malignant transformation of mesenchymal stem cells significantly affect the functionality of these cells. [Conclusion] Definitive conclusions cannot be made by these studies because limited numbers of patients were included. Studies clarifying these results are expected in the near future.
Keywords: Mesenchymal stem cells; Regenerative rehabilitation.
Figures
References
-
- Volarevic V, Nurkovic J, Arsenijevic N, et al. : Concise review: therapeutic potential of mesenchymal stem cells for the treatment of acute liver failure and cirrhosis. Stem Cells, 2014, 32: 2818–2823. - PubMed
-
- Syková E, Homola A, Mazanec R, et al. : Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transplant, 2006, 15: 675–687. - PubMed
-
- Geffner LF, Santacruz P, Izurieta M, et al. : Administration of autologous bone marrow stem cells into spinal cord injury patients via multiple routes is safe and improves their quality of life: comprehensive case studies. Cell Transplant, 2008, 17: 1277–1293. - PubMed
-
- De Coppi P: Regenerative medicine for congenital malformations. J Pediatr Surg, 2013, 48: 273–280. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous