Clinical Efficacy of Ovarian Cancer Screening
- PMID: 27390606
- PMCID: PMC4934039
- DOI: 10.7150/jca.14615
Clinical Efficacy of Ovarian Cancer Screening
Abstract
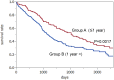
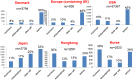
Various trials of ovarian cancer screening programs have been reported worldwide. In 2011, one of the most famous papers indicated that annual screening using CA125/transvaginal sonography (TVS) did not reduce ovarian cancer mortality in the United States of America (USA). To investigate the validity of ovarian cancer screening, we verified the analyses of previous reports. At first, we obtained the USA datasets that were used for the analyses and identified many patients in whom cancers were accidentally detected several years after the screening period. We thus performed a new prognostic comparison between the screening group (cancers that were detected through screening or within one year after screening) and the control group (cancers that were found more than one year after screening, without screening, or in the original control group). The results showed that the prognoses of the screening group were significantly better than those of the control group (p=0.0017). In addition, the screening group contained significantly fewer stage IV cases than the control group (p=0.005). In another screening in the United Kingdom, ovarian cancer was detected at a relatively earlier stage (stage I/II: 44%), while the rate of stage IV detection was low (4%). Very recently, this team showed significant difference in the rates with and without screening (p=0.021) when prevalent cases were excluded and indicated the delayed effect of screening. These results contrasted with the USA data. In other studies in the USA and Japan, annual screening was also associated with a decreased stage at detection. New histopathological, molecular and genetic studies have recently provided two categories of ovarian carcinogenesis. Type I carcinomas are slow-growing neoplasms that often develop from benign ovarian cysts. Type II carcinomas are high-grade clinically aggressive neoplasms. The rate of type II carcinomas is significantly higher in Europe and the USA than in Asia (p<0.001). Conversely, type I carcinomas are relatively common in Asia. These data theoretically imply that annual screening would be more effective in Asia.
Keywords: CA125; Kaplan-Meier survival curve.; ovarian cancer screening; transvaginal sonography.
Conflict of interest statement
Competing Interests: The authors have no financial conflicts of interest relevant to the present work to declare.
Figures

References
-
- Buys SS, Partridge E, Greene MH. et al. Ovarian cancer screening in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial: findings from the initial screen of a randomized trial. Am J Obstet Gynecol. 2005;193:1630–9. - PubMed
-
- Buys SS, Patridge E, Black A. et al. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and ovarian (PLCO) cancer screening randomized controlled trial. JAMA. 2011;305:2295–303. - PubMed
-
- Menon U, Gentry-Maharaj A, Hallett R. et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) Lancet Oncol. 2009;10:327–40. - PubMed
-
- Sharma A, Gentr-Maharaj A, Burnell M. et al. Assessing the malignant potential of ovarian inclusion cysts in postmenomausal women within the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a prospective cohort study. Gynecol Oncol. 2012;119:207–19. - PubMed
-
- Sharma A, Apostolidou S, Burnell M. et al. Risk of epithelial ovarian cancer in asymptomatic women with ultrasound-detected ovarian masses: a prospective cohort study within the UK collaborative trial of ovarian cancer screening (UKCTOCS) Ultrasound Obstet Gynecol. 2012;40:338–44. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

