Microglial Contact Prevents Excess Depolarization and Rescues Neurons from Excitotoxicity
- PMID: 27390772
- PMCID: PMC4916329
- DOI: 10.1523/ENEURO.0004-16.2016
Microglial Contact Prevents Excess Depolarization and Rescues Neurons from Excitotoxicity
Abstract
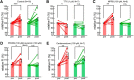
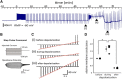
Microglia survey and directly contact neurons in both healthy and damaged brain, but the mechanisms and functional consequences of these contacts are not yet fully elucidated. Combining two-photon imaging and patch clamping, we have developed an acute experimental model for studying the role of microglia in CNS excitotoxicity induced by neuronal hyperactivity. Our model allows us to simultaneously examine the effects of repetitive supramaximal stimulation on axonal morphology, neuronal membrane potential, and microglial migration, using cortical brain slices from Iba-1 eGFP mice. We demonstrate that microglia exert an acute and highly localized neuroprotective action under conditions of neuronal hyperactivity. Evoking repetitive action potentials in individual layer 2/3 pyramidal neurons elicited swelling of axons, but not dendrites, which was accompanied by a large, sustained depolarization of soma membrane potential. Microglial processes migrated to these swollen axons in a mechanism involving both ATP and glutamate release via volume-activated anion channels. This migration was followed by intensive microglial wrapping of affected axons and, in some cases, the removal of axonal debris that induced a rapid soma membrane repolarization back to resting potentials. When the microglial migration was pharmacologically blocked, the activity-induced depolarization continued until cell death ensued, demonstrating that the microglia-axon contact served to prevent pathological depolarization of the soma and maintain neuronal viability. This is a novel aspect of microglia surveillance: detecting, wrapping, and rescuing neuronal soma from damage due to excessive activity.
Keywords: ATP release; axonal swelling; excitotoxicty; microglia; neuronal rescue.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
