Colorectal cancer health services research study protocol: the CCR-CARESS observational prospective cohort project
- PMID: 27391216
- PMCID: PMC4939051
- DOI: 10.1186/s12885-016-2475-y
Colorectal cancer health services research study protocol: the CCR-CARESS observational prospective cohort project
Abstract
Background: Colorectal cancers are one of the most common forms of malignancy worldwide. But two significant areas of research less studied deserve attention: health services use and development of patient stratification risk tools for these patients.
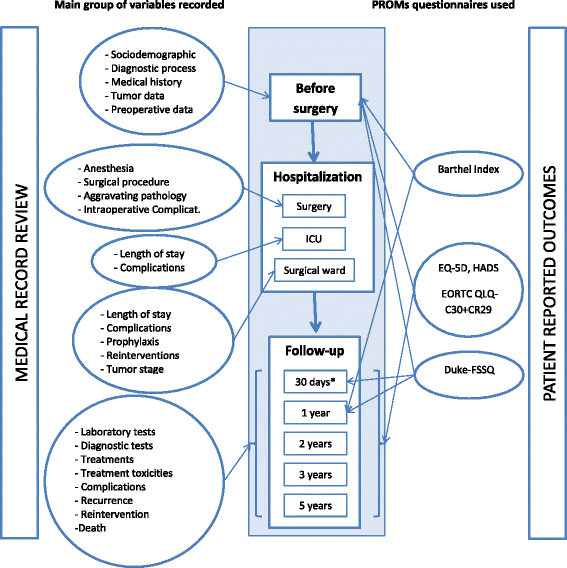
Design: a prospective multicenter cohort study with a follow up period of up to 5 years after surgical intervention. Participant centers: 22 hospitals representing six autonomous communities of Spain. Participants/Study population: Patients diagnosed with colorectal cancer that have undergone surgical intervention and have consented to participate in the study between June 2010 and December 2012. Variables collected include pre-intervention background, sociodemographic parameters, hospital admission records, biological and clinical parameters, treatment information, and outcomes up to 5 years after surgical intervention. Patients completed the following questionnaires prior to surgery and in the follow up period: EuroQol-5D, EORTC QLQ-C30 (The European Organization for Research and Treatment of Cancer quality of life questionnaire) and QLQ-CR29 (module for colorectal cancer), the Duke Functional Social Support Questionnaire, the Hospital Anxiety and Depression Scale, and the Barthel Index. The main endpoints of the study are mortality, tumor recurrence, major complications, readmissions, and changes in health-related quality of life at 30 days and at 1, 2, 3 and 5 years after surgical intervention.
Statistical analysis: In relation to the different endpoints, predictive models will be used by means of multivariate logistic models, Cox or linear mixed-effects regression models. Simulation models for the prediction of discrete events in the long term will also be used, and an economic evaluation of different treatment strategies will be performed through the use of generalized linear models.
Discussion: The identification of potential risk factors for adverse events may help clinicians in the clinical decision making process. Also, the follow up by 5 years of this large cohort of patients may provide useful information to answer different health services research questions.
Trial registration: ClinicalTrials.gov Identifier: NCT02488161 . Registration date: June 16, 2015.
Keywords: Clinical prediction rule; Colon cancer; Health services research; Rectal cancer.
Figures


References
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

