Multiplexed Spectral Imaging of 120 Different Fluorescent Labels
- PMID: 27391327
- PMCID: PMC4938436
- DOI: 10.1371/journal.pone.0158495
Multiplexed Spectral Imaging of 120 Different Fluorescent Labels
Abstract
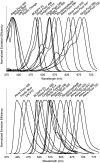
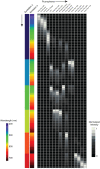
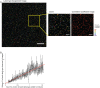
The number of fluorescent labels that can unambiguously be distinguished in a single image when acquired through band pass filters is severely limited by the spectral overlap of available fluorophores. The recent development of spectral microscopy and the application of linear unmixing algorithms to spectrally recorded image data have allowed simultaneous imaging of fluorophores with highly overlapping spectra. However, the number of distinguishable fluorophores is still limited by the unavoidable decrease in signal to noise ratio when fluorescence signals are fractionated over multiple wavelength bins. Here we present a spectral image analysis algorithm to greatly expand the number of distinguishable objects labeled with binary combinations of fluorophores. Our algorithm utilizes a priori knowledge about labeled specimens and imposes a binary label constraint on the unmixing solution. We have applied our labeling and analysis strategy to identify microbes labeled by fluorescence in situ hybridization and here demonstrate the ability to distinguish 120 differently labeled microbes in a single image.
Conflict of interest statement
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

