Impaired DNA double-strand break repair contributes to the age-associated rise of genomic instability in humans
- PMID: 27391797
- PMCID: PMC5071568
- DOI: 10.1038/cdd.2016.65
Impaired DNA double-strand break repair contributes to the age-associated rise of genomic instability in humans
Abstract
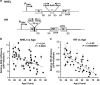
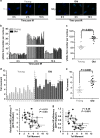
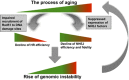
Failing to repair DNA double-strand breaks by either nonhomologous end joining (NHEJ) or homologous recombination (HR) poses a threat to genome integrity, and may have roles in the onset of aging and age-related diseases. Recent work indicates an age-related decrease of NHEJ efficiency in mouse models, but whether NHEJ and HR change with age in humans and the underlying mechanisms of such a change remain uncharacterized. Here, using 50 eyelid fibroblast cell lines isolated from healthy donors at the age of 16-75 years, we demonstrate that the efficiency and fidelity of NHEJ, and the efficiency of HR decline with age, leading to increased IR sensitivity in cells isolated from old donors. Mechanistic analysis suggests that decreased expression of XRCC4, Lig4 and Lig3 drives the observed, age-associated decline of NHEJ efficiency and fidelity. Restoration of XRCC4 and Lig4 significantly promotes the fidelity and efficiency of NHEJ in aged fibroblasts. In contrast, essential HR-related factors, such as Rad51, do not change in expression level with age, but Rad51 exhibits a slow kinetics of recruitment to DNA damage sites in aged fibroblasts. Further rescue experiments indicate that restoration of XRCC4 and Lig4 may suppress the onset of stress-induced premature cellular senescence, suggesting that improving NHEJ efficiency and fidelity by targeting the NHEJ pathway holds great potential to delay aging and mitigate aging-related pathologies.
Figures





References
-
- Tigges J, Krutmann J, Fritsche E, Haendeler J, Schaal H, Fischer JW et al. The hallmarks of fibroblast ageing. Mech Ageing Dev 2014; 138: 26–44. - PubMed
-
- Dolle ME, Giese H, Hopkins CL, Martus HJ, Hausdorff JM, Vijg J. Rapid accumulation of genome rearrangements in liver but not in brain of old mice. Nat Genet 1997; 17: 431–434. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

