Mechanical Ventilation Alters the Development of Staphylococcus aureus Pneumonia in Rabbit
- PMID: 27391952
- PMCID: PMC4938582
- DOI: 10.1371/journal.pone.0158799
Mechanical Ventilation Alters the Development of Staphylococcus aureus Pneumonia in Rabbit
Abstract
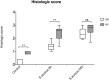
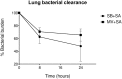
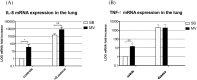
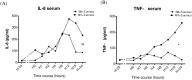
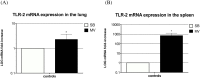
Ventilator-associated pneumonia (VAP) is common during mechanical ventilation (MV). Beside obvious deleterious effects on muco-ciliary clearance, MV could adversely shift the host immune response towards a pro-inflammatory pattern through toll-like receptor (TLRs) up-regulation. We tested this hypothesis in a rabbit model of Staphylococcus aureus VAP. Pneumonia was caused by airway challenge with S. aureus, in either spontaneously breathing (SB) or MV rabbits (n = 13 and 17, respectively). Pneumonia assessment regarding pulmonary and systemic bacterial burden, as well as inflammatory response was done 8 and 24 hours after S. aureus challenge. In addition, ex vivo stimulations of whole blood taken from SB or MV rabbits (n = 7 and 5, respectively) with TLR2 agonist or heat-killed S. aureus were performed. Data were expressed as mean±standard deviation. After 8 hours of infection, lung injury was more severe in MV animals (1.40±0.33 versus [vs] 2.40±0.55, p = 0.007), along with greater bacterial concentrations (6.13±0.63 vs. 4.96±1.31 colony forming units/gram, p = 0.002). Interleukin (IL)-8 and tumor necrosis factor (TNF)-αserum concentrations reached higher levels in MV animals (p = 0.010). Whole blood obtained from MV animals released larger amounts of cytokines if stimulated with TLR2 agonist or heat-killed S. aureus (e.g., TNF-α: 1656±166 vs. 1005±89; p = 0.014). Moreover, MV induced TLR2 overexpression in both lung and spleen tissue. MV hastened tissue injury, impaired lung bacterial clearance, and promoted a systemic inflammatory response, maybe through TLR2 overexpression.
Conflict of interest statement
Figures





References
-
- Chastre J, Fagon JY. Ventilator-associated pneumonia. American journal of respiratory and critical care medicine. 2002;165(7):867–903. - PubMed
-
- Mourgeon E, Isowa N, Keshavjee S, Zhang X, Slutsky AS, Liu M. Mechanical stretch stimulates macrophage inflammatory protein-2 secretion from fetal rat lung cells. Am J Physiol Lung Cell Mol Physiol. 2000;279(4):L699–706. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

