Induction of Suppressor Cells and Increased Tumor Growth following Chronic Psychosocial Stress in Male Mice
- PMID: 27391954
- PMCID: PMC4938385
- DOI: 10.1371/journal.pone.0159059
Induction of Suppressor Cells and Increased Tumor Growth following Chronic Psychosocial Stress in Male Mice
Abstract
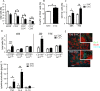
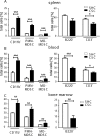
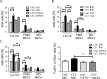
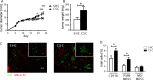
To study the impact of psychosocial stress on the immune system, male mice were subjected to chronic subordinate colony housing (CSC), a preclinically validated mouse model for chronic psychosocial stress. CSC substantially affected the cell composition of the bone marrow, blood, and spleen by inducing myelopoiesis and enhancing the frequency of regulatory T cells in the CD4 population. Expansion of the myeloid cell compartment was due to cells identified as immature inflammatory myeloid cells having the phenotype of myeloid-derived suppressor cells of either the granulocytic or the monocytic type. Catecholaminergic as well as TNF signaling were implicated in these CSC-induced cellular shifts. Although the frequency of regulatory cells was enhanced following CSC, the high capacity for inflammatory cytokine secretion of total splenocytes indicated an inflammatory immune status in CSC mice. Furthermore, CSC enhanced the suppressive activity of bone marrow-derived myeloid-derived suppressor cells towards proliferating T cells. In line with the occurrence of suppressor cell types such as regulatory T cells and myeloid-derived suppressor cells, transplanted syngeneic fibrosarcoma cells grew better in CSC mice than in controls, a process accompanied by pronounced angiogenesis and clustering of immature myeloid cells in the tumor tissue. In addition, tumor implantation after CSC reinforced the CSC-induced increase in myeloid-derived suppressor cells and regulatory T cell frequencies while the CSC-induced cellular changes eased off in mice without tumor. Together, our data suggest a role for suppressor cells such as regulatory T cells and myeloid-derived suppressor cells in the enhanced tumor growth after chronic psychosocial stress.
Conflict of interest statement
Figures

References
-
- Selye H. A syndrome produced by diverse nocuous agents. 1936. J Neuropsychiatry Clin Neurosci 1998;10(2):230–1. - PubMed
-
- Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proc Natl Acad Sci U S A 2013. October 8;110(41):16574–9. 10.1073/pnas.1310655110 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

