Bi-allelic Mutations in KLHL7 Cause a Crisponi/CISS1-like Phenotype Associated with Early-Onset Retinitis Pigmentosa
- PMID: 27392078
- PMCID: PMC5005468
- DOI: 10.1016/j.ajhg.2016.05.026
Bi-allelic Mutations in KLHL7 Cause a Crisponi/CISS1-like Phenotype Associated with Early-Onset Retinitis Pigmentosa
Erratum in
-
Bi-allelic Mutations in KLHL7 Cause a Crisponi/CISS1-like Phenotype Associated with Early-Onset Retinitis Pigmentosa.Am J Hum Genet. 2018 Apr 5;102(4):713. doi: 10.1016/j.ajhg.2018.03.020. Am J Hum Genet. 2018. PMID: 29625027 Free PMC article. No abstract available.
Abstract
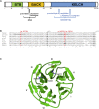
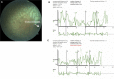
Crisponi syndrome (CS)/cold-induced sweating syndrome type 1 (CISS1) is a very rare autosomal-recessive disorder characterized by a complex phenotype with high neonatal lethality, associated with the following main clinical features: hyperthermia and feeding difficulties in the neonatal period, scoliosis, and paradoxical sweating induced by cold since early childhood. CS/CISS1 can be caused by mutations in cytokine receptor-like factor 1 (CRLF1). However, the physiopathological role of CRLF1 is still poorly understood. A subset of CS/CISS1 cases remain yet genetically unexplained after CRLF1 sequencing. In five of them, exome sequencing and targeted Sanger sequencing identified four homozygous disease-causing mutations in kelch-like family member 7 (KLHL7), affecting the Kelch domains of the protein. KLHL7 encodes a BTB-Kelch-related protein involved in the ubiquitination of target proteins for proteasome-mediated degradation. Mono-allelic substitutions in other domains of KLHL7 have been reported in three families affected by a late-onset form of autosomal-dominant retinitis pigmentosa. Retinitis pigmentosa was also present in two surviving children reported here carrying bi-allelic KLHL7 mutations. KLHL7 mutations are thus associated with a more severe phenotype in recessive than in dominant cases. Although these data further support the pathogenic role of KLHL7 mutations in a CS/CISS1-like phenotype, they do not explain all their clinical manifestations and highlight the high phenotypic heterogeneity associated with mutations in KLHL7.
Copyright © 2016 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Dagoneau N., Bellais S., Blanchet P., Sarda P., Al-Gazali L.I., Di Rocco M., Huber C., Djouadi F., Le Goff C., Munnich A., Cormier-Daire V. Mutations in cytokine receptor-like factor 1 (CRLF1) account for both Crisponi and cold-induced sweating syndromes. Am. J. Hum. Genet. 2007;80:966–970. - PMC - PubMed
-
- Piras R., Chiappe F., Torraca I.L., Buers I., Usala G., Angius A., Akin M.A., Basel-Vanagaite L., Benedicenti F., Chiodin E. Expanding the mutational spectrum of CRLF1 in Crisponi/CISS1 syndrome. Hum. Mutat. 2014;35:424–433. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

