Flagellin-rPAc vaccine inhibits biofilm formation but not proliferation of S. mutans
- PMID: 27392114
- PMCID: PMC5137529
- DOI: 10.1080/21645515.2016.1203496
Flagellin-rPAc vaccine inhibits biofilm formation but not proliferation of S. mutans
Abstract
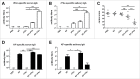
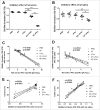
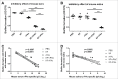
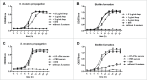
As the main etiologic bacterium of dental caries, Streptococcus mutans (S. mutans) has been considered as the primary object of vaccine research. We previously constructed a recombinant flagellin-rPAc fusion protein (KF-rPAc) that consists of an alanine-rich region to proline-rich region fragment of PAc (rPAc) from S. mutans and flagellin KF from E.coli K12 strain. Intranasal (i.n) immunization of KF-rPAc could induce high level of rPAc-specific antibody responses and offer robust protection against dental caries. In caries development, biofilm formation was considered as the necessary process involved. As PAc possesses other activities besides affecting adherence of S. mutans to salivary glycoproteins, we wondered whether rPAc-specific antibody responses induced by KF-rPAc could inhibit biofilm formation. Hence, in the present study, a simple and convenient in vitro biofilm model of S. mutans was constructed without saliva pre-coated. Both serum and saliva from KF-rPAc immunized rats significantly inhibited biofilm formation. Moreover, with the presence of serum or saliva, the biofilm formation is negatively correlated with the level of rPAc-specific antibody, and positively correlated with caries scores in rat. Moreover, in immunized mice, the level of rPAc-specific antibody also negatively correlated with the biofilm formation. Unlike ampicillin, serum of KF-rPAc immunized mice only inhibited biofilm formation but not proliferation. All together, we discovered that besides the well known blocking adherence of S. mutans to salivary glycoproteins by rPAc-specific antibody, flagellin-rPAc vaccine could also protects tooth from caries by inhibiting biofilm structure formation in between bacteria.
Keywords: PAc; Streptococcus mutans; biofilm; dental caries; flagellin; mucosal; vaccine.
Figures

Similar articles
-
Flagellin-PAc fusion protein is a high-efficacy anti-caries mucosal vaccine.J Dent Res. 2012 Oct;91(10):941-7. doi: 10.1177/0022034512457684. Epub 2012 Aug 14. J Dent Res. 2012. PMID: 22895510
-
Flagellin-PAc Fusion Protein Inhibits Progression of Established Caries.J Dent Res. 2015 Jul;94(7):955-60. doi: 10.1177/0022034515582224. Epub 2015 Apr 16. J Dent Res. 2015. PMID: 25883108
-
Immunogenicity and protective effect against oral colonization by Streptococcus mutans of synthetic peptides of a streptococcal surface protein antigen.J Immunol. 1991 Jan 1;146(1):332-6. J Immunol. 1991. PMID: 1984447
-
Salivary IgA enhancement strategy for development of a nasal-spray anti-caries mucosal vaccine.Sci China Life Sci. 2013 May;56(5):406-13. doi: 10.1007/s11427-013-4473-5. Epub 2013 May 1. Sci China Life Sci. 2013. PMID: 23633072 Review.
-
A Caries Vaccine? The state of the science of immunization against dental caries.Caries Res. 2004 May-Jun;38(3):230-5. doi: 10.1159/000077759. Caries Res. 2004. PMID: 15153693 Review.
Cited by
-
The Combinations Chitosan-Pam3CSK4 and Chitosan-Monophosphoryl Lipid A: Promising Immune-Enhancing Adjuvants for Anticaries Vaccine PAc.Infect Immun. 2019 Nov 18;87(12):e00651-19. doi: 10.1128/IAI.00651-19. Print 2019 Dec. Infect Immun. 2019. PMID: 31527122 Free PMC article.
-
Second-generation Flagellin-rPAc Fusion Protein, KFD2-rPAc, Shows High Protective Efficacy against Dental Caries with Low Potential Side Effects.Sci Rep. 2017 Sep 11;7(1):11191. doi: 10.1038/s41598-017-10247-8. Sci Rep. 2017. PMID: 28894188 Free PMC article.
-
An optimized caries model of Streptococcus mutans in rats and its application for evaluating prophylactic vaccines.Hum Vaccin Immunother. 2024 Dec 31;20(1):2345943. doi: 10.1080/21645515.2024.2345943. Epub 2024 May 17. Hum Vaccin Immunother. 2024. PMID: 38757492 Free PMC article.
-
A Nanoparticle-Based Anticaries Vaccine Enhances the Persistent Immune Response To Inhibit Streptococcus mutans and Prevent Caries.Microbiol Spectr. 2023 Mar 28;11(2):e0432822. doi: 10.1128/spectrum.04328-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36976019 Free PMC article.
References
-
- Forester H, Hunter N, Knox KW. Characteristics of a high molecular weight extracellular protein of Streptococcus mutans. J Gen Microbiol 1983; 129:2779-88; PMID:6415234 - PubMed
-
- Russell RR. Wall-associated protein antigens of Streptococcus mutans. J Gen Microbiol 1979; 114:109-15; PMID:118231; http://dx.doi.org/10.1099/00221287-114-1-109 - DOI - PubMed
-
- Vonderviszt F, Aizawa S, Namba K. Role of the disordered terminal regions of flagellin in filament formation and stability. J Mol Biol 1991; 221:1461-74; PMID:1942062; http://dx.doi.org/10.1016/0022-2836(91)90946-4 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
