The Anti-Prion Antibody 15B3 Detects Toxic Amyloid-β Oligomers
- PMID: 27392850
- PMCID: PMC5044783
- DOI: 10.3233/JAD-150882
The Anti-Prion Antibody 15B3 Detects Toxic Amyloid-β Oligomers
Abstract
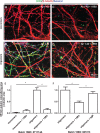
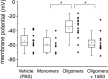
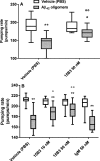
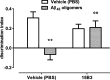
15B3 is a monoclonal IgM antibody that selectively detects pathological aggregates of the prion protein (PrP). We report the unexpected finding that 15B3 also recognizes oligomeric but not monomeric forms of amyloid-β (Aβ)42, an aggregating peptide implicated in the pathogenesis of Alzheimer's disease (AD). The 15B3 antibody: i) inhibits the binding of synthetic Aβ42 oligomers to recombinant PrP and neuronal membranes; ii) prevents oligomer-induced membrane depolarization; iii) antagonizes the inhibitory effects of oligomers on the physiological pharyngeal contractions of the nematode Caenorhabditis elegans; and iv) counteracts the memory deficits induced by intracerebroventricular injection of Aβ42 oligomers in mice. Thus this antibody binds to pathologically relevant forms of Aβ, and offers a potential research, diagnostic, and therapeutic tool for AD.
Keywords: 15B3 antibody; Alzheimer’s disease; amyloid beta-protein (1– 42); oligomers; prion protein; prions.
Figures




References
-
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH (2005) Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci 8, 79–84. - PubMed
-
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL (1998) Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A 95, 6448–6453. - PMC - PubMed
-
- Walsh DM (2002) Naturally secreted oligomers of amyloid [beta] protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535–539. - PubMed
-
- Balducci C, Beeg M, Stravalaci M, Bastone A, Sclip A, Biasini E, Tapella L, Colombo L, Manzoni C, Borsello T, Chiesa R, Gobbi M, Salmona M, Forloni G (2010) Synthetic amyloid-beta oligomers impair long-term memory independently of cellular prion protein. Proc Natl Acad Sci U S A 107, 2295–2300. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

