Effects of atorvastatin on renal function in patients with dyslipidemia and chronic kidney disease: assessment of clinical usefulness in CKD patients with atorvastatin (ASUCA) trial
- PMID: 27392909
- PMCID: PMC5486454
- DOI: 10.1007/s10157-016-1304-6
Effects of atorvastatin on renal function in patients with dyslipidemia and chronic kidney disease: assessment of clinical usefulness in CKD patients with atorvastatin (ASUCA) trial
Abstract
Background: Dyslipidemia is a risk factor for the progression of chronic kidney disease (CKD). While conventional lipid lowering therapy provides a benefit to CKD management, the effect of statins on eGFR remains unclear.
Methods: A prospective, multi-center, open-labeled, randomized trial. Total of 349 CKD patients with hyperlipidemia were randomized into 2 groups, and followed for 2 years. Group A included patients who were treated with atorvastatin. Group C were treated with conventional lipid lowering drugs other than statin. Primary endpoint was changes in eGFR. Secondary endpoints included changes in urinary albumin excretion, serum LDL-C, serum triglyceride, cardio-vascular events and all-cause mortality.
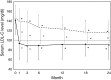
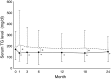
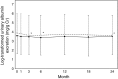
Results: As the primary endpoint, eGFR decreased by 2.3 ml/min/1.73 m2 in Group A and by 2.6 ml/min/1.73 m2 in Group C, indicating that there was no difference in change of eGFR between the two groups. As secondary endpoints, atorvastatin succeeded to reduce serum LDL-C level significantly and rapidly, but conventional therapy did not. In fact, mean LDL-C level did not reach the target level of 100 mg/dl in Group C. Serum triglyceride was lowered only by atorvastatin, but not conventional drugs. The number of cardiovascular events and all-cause mortality did not differ between in two groups.
Conclusion: The ASUCA (Assessment of Clinical Usefulness in CKD Patients with Atorvastatin) trial demonstrated that atorvastatin failed to exhibit reno-protections compared to conventional therapy in Japanese patients with dyslipidemia and CKD. It would be due in part to the ability of atorvastatin to more potently reduce serum LDL and triglycerides compared to conventional therapy.
Keywords: Chronic kidney disease (CKD); Hyperlipidemia; Low-density lipoprotein cholesterol (LDL-C); Reno-protective effect; Statins.
Conflict of interest statement
Source funding
The ASUCA trial was funded by Department of EBM Research Institute of Advancement of Clinical and Translational Science Kyoto University Hospital with an unrestricted grant from Pfizer Japan.
Conflict of interest
Honoraria: Genjiro Kimura (Takeda, Daiichi-Sankyo, Novartis and Taisho-Toyama companies), Masato Ksahara (Pfizer, Daiichi-Sankyo, Fuji companies), Kenji Ueshima (Pfizer company).
Grants received: Genjiro Kimura (Japan Labour Health and Welfare Organization).
Figures


References
-
- Shepherd J, Kastelein JJ, Bittner V, Deedwania P, Breazna A, Dobson S, Wilson DJ, Zuckerman A, Wenger NK, Treating to New Targets Investigators Effect of intensive lipid lowering with atorvastatin on renal function in patients with coronary heart disease: the treating to new targets (TNT) study. Clin J Am Soc Nephrol. 2007;2(6):1131–1139. doi: 10.2215/CJN.04371206. - DOI - PubMed
-
- Athyros VG, Mikhailidis DP, Liberopoulos EN, Kakafika AI, Karagiannis A, Papageorgiou AA, Tziomalos K, Ganotakis ES, Elisaf M. Effect of statin treatment on renal function and serum uric acid levels and their relation to vascular events in patients with coronary heart disease and metabolic syndrome: a subgroup analysis of the GREek Atorvastatin and Coronary heart disease Evaluation (GREACE) Study. Nephrol Dial Transpl. 2007;22(1):118–127. doi: 10.1093/ndt/gfl538. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

