Effect of mutations to amino acid A301 and F361 in thermostability and catalytic activity of the β-galactosidase from Bacillus subtilis VTCC-DVN-12-01
- PMID: 27393145
- PMCID: PMC4938916
- DOI: 10.1186/s12858-016-0070-0
Effect of mutations to amino acid A301 and F361 in thermostability and catalytic activity of the β-galactosidase from Bacillus subtilis VTCC-DVN-12-01
Abstract
Background: Beta-galactosidase (EC 3.2.1.23), a commercially important enzyme, catalyses the hydrolysis of β-1,3- and β-1,4-galactosyl bonds of polymer or oligosaccharidesas well as transglycosylation of β-galactopyranosides. Due to catalytic properties; β-galactosidase might be useful in the milk industry to hydrolyze lactose and produce prebiotic GOS. The purpose of this study is to characterize β-galactosidase mutants from B. subtilis.
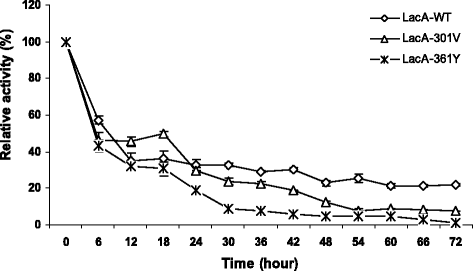
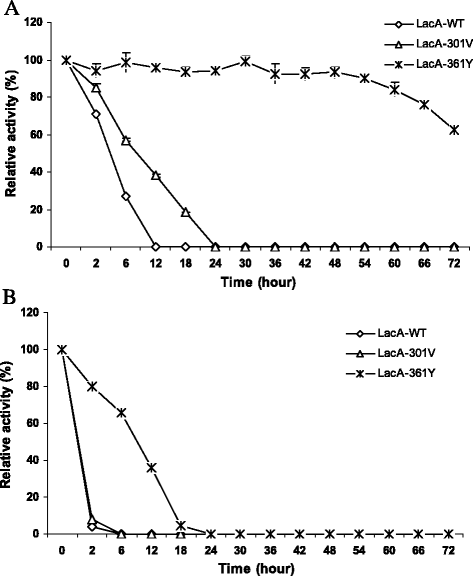
Results: Using error prone rolling circle amplification (epRCA) to characterize some random mutants of the β-galactosidase (LacA) from B. subtilisVTCC-DVN-12-01, amino acid A301 and F361 has been demonstrated significantly effect on hydrolysis activity of LacA. Mutants A301V and F361Y had markedly reduced hydrolysis activity to 23.69 and 43.22 %, respectively. Mutants the site-saturation of A301 reduced catalysis efficiency of LacA to 20-50 %, while the substitution of F361 by difference amino acids (except tyrosine) lost all of enzymatic activity, indicating that A301 and F361 are important for the catalytic function. Interestingly, the mutant F361Y exhibited enhanced significantly thermostability of enzyme at 45-50 °C. At 45 °C, LacA-361Y retained over 93 % of its original activity for 48 h of incubation, whereas LacA-WT and LacA-301Vwere lost completely after 12 and 24 h of incubation, respectively. The half-life times of LacA-361Y and LacA-301 V were about 26.8 and 2.4 times higher, respectively, in comparison to the half-life time of LacA-WT. At temperature optimum 50 °C, LacA-361Y shows more stable than LacA-WT and LacA-301 V, retaining 79.88 % of its original activities after 2 h of incubation, while the LacA-WT and LacA-301 V lost all essential activities. The half-life time of LacA-361Y was higher 12.7 and 9.39 times than that of LacA-WT and LacA-301 V, respectively. LacA-WT and mutant enzymes were stability at pH 5-9, retained over 90 % activity for 72 h of incubation at 30 °C. However, LacA-WT showed a little bit more stability than LacA-301 V and LacA-361Y at pH 4.
Conclusions: Our findings demonstrated that the amino acids A301V and F361 play important role in hydrolysis activity of β -galactosidase from B. subtilis. Specially, amino acid F361 had noteworthy effect on both catalytic and thermostability of LacA enzyme, suggesting that F361 is responsible for functional requirement of the GH42 family.
Keywords: Bacillus subtilis; Catalytic activity; Error prone rolling circle amplification; Escherichia coli; Mutants; β-Galactosidase.
Figures



Similar articles
-
[Cloning, expression, directed evolution in vitro and structural simulation of β-glycosidase from Bacillus subtilis].Wei Sheng Wu Xue Bao. 2015 Oct 4;55(10):1273-83. Wei Sheng Wu Xue Bao. 2015. PMID: 26939455 Chinese.
-
Characterization of a β-galactosidase from Bacillus subtilis with transgalactosylation activity.Int J Biol Macromol. 2018 Dec;120(Pt A):279-287. doi: 10.1016/j.ijbiomac.2018.07.116. Epub 2018 Jul 21. Int J Biol Macromol. 2018. PMID: 30036621
-
Production, purification, and characterization of a potential thermostable galactosidase for milk lactose hydrolysis from Bacillus stearothermophilus.J Dairy Sci. 2008 May;91(5):1751-8. doi: 10.3168/jds.2007-617. J Dairy Sci. 2008. PMID: 18420605
-
Experimental evolution of Ebg enzyme provides clues about the evolution of catalysis and to evolutionary potential.FEMS Microbiol Lett. 1999 May 1;174(1):1-8. doi: 10.1111/j.1574-6968.1999.tb13542.x. FEMS Microbiol Lett. 1999. PMID: 10234816 Review.
-
Sources, properties and suitability of new thermostable enzymes in food processing.Crit Rev Food Sci Nutr. 2006;46(3):197-205. doi: 10.1080/10408690590957296. Crit Rev Food Sci Nutr. 2006. PMID: 16527752 Review.
Cited by
-
Valorization of whey-based side streams for microbial biomass, molecular hydrogen, and hydrogenase production.Appl Microbiol Biotechnol. 2023 Jul;107(14):4683-4696. doi: 10.1007/s00253-023-12609-x. Epub 2023 Jun 8. Appl Microbiol Biotechnol. 2023. PMID: 37289241
References
-
- Haider T, Husain Q. β-Galactosidase by bioaffinity adsorption on immobilization of concanavalin A lay red calcium alginate-starch hybrid beads for the hyrolysis of lactose from whey/milk. Int Dairy J. 2009;19:172–7. doi: 10.1016/j.idairyj.2008.10.005. - DOI
-
- Neri DFM, Balcao VM, Carneiro-da-Cunha MG, Carvalho LB, Jr, Teixeira JA. Immobilization of β-galactosidase from Kluyveromyces lactis onto a polysiloxane–polyvinyl alcohol magnetic (mPOS–PVA) composite for lactose hydrolysis. Catal Comm. 2008;4:2334–9. doi: 10.1016/j.catcom.2008.05.022. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

