High-fat diet-induced brain region-specific phenotypic spectrum of CNS resident microglia
- PMID: 27393312
- PMCID: PMC4992033
- DOI: 10.1007/s00401-016-1595-4
High-fat diet-induced brain region-specific phenotypic spectrum of CNS resident microglia
Abstract
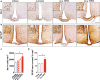
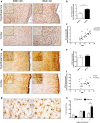
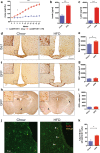
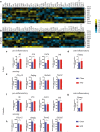
Diets high in fat (HFD) are known to cause an immune response in the periphery as well as the central nervous system. In peripheral adipose tissue, this immune response is primarily mediated by macrophages that are recruited to the tissue. Similarly, reactivity of microglia, the innate immune cells of the brain, has been shown to occur in the hypothalamus of mice fed a high-fat diet. To characterize the nature of the microglial response to diets high in fat in a temporal fashion, we studied the phenotypic spectrum of hypothalamic microglia of mice fed high-fat diet for 3 days and 8 weeks by assessing their tissue reaction and inflammatory signature. While we observed a significant increase in Iba1+ myeloid cells and a reaction of GFAP+ astrocytes in the hypothalamus after 8 weeks of HFD feeding, we found the hypothalamic myeloid cell reaction to be limited to endogenous microglia and not mediated by infiltrating myeloid cells. Moreover, obese humans were found to present with signs of hypothalamic gliosis and exacerbated microglia dystrophy, suggesting a targeted microglia response to diet in humans as well. Notably, the glial reaction occurring in the mouse hypothalamus was not accompanied by an increase in pro-inflammatory cytokines, but rather by an anti-inflammatory reaction. Gene expression analyses of isolated microglia not only confirmed this observation, but also revealed a downregulation of microglia genes important for sensing signals in the microenvironment. Finally, we demonstrate that long-term exposure of microglia to HFD in vivo does not impair the cell's ability to respond to additional stimuli, like lipopolysaccharide. Taken together, our findings support the notion that microglia react to diets high in fat in a region-specific manner in rodents as well as in humans; however, this response changes over time as it is not exclusively pro-inflammatory nor does exposure to HFD prime microglia in the hypothalamus.
Keywords: Glia; High-fat diet; Metabolism; Microglia.
Figures


References
-
- Baquedano E, Ruiz-Lopez AM, Sustarsic EG, Herpy J, List EO, Chowen JA, Frago LM, Kopchick JJ, Argente J. The absence of GH signaling affects the susceptibility to high-fat diet-induced hypothalamic inflammation in male mice. Endocrinology. 2014;155:4856–4867. doi: 10.1210/en.2014-1367. - DOI - PubMed
-
- Vom Berg J, Prokop S, Miller KR, Obst J, Kälin RE, Lopategui-Cabezas I, Wegner A, Mair F, Schipke CG, Peters O, Winter Y, Becher B, Heppner FL. Inhibition of IL-12/IL-23 signaling reduces Alzheimer’s disease-like pathology and cognitive decline. Nat Med. 2012;18:1812–1819. doi: 10.1038/nm.2965. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

