SNHG16 is regulated by the Wnt pathway in colorectal cancer and affects genes involved in lipid metabolism
- PMID: 27396952
- PMCID: PMC5423192
- DOI: 10.1016/j.molonc.2016.06.003
SNHG16 is regulated by the Wnt pathway in colorectal cancer and affects genes involved in lipid metabolism
Abstract
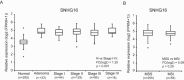
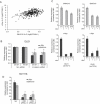
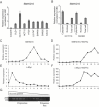
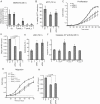
It is well established that lncRNAs are aberrantly expressed in cancer where they have been shown to act as oncogenes or tumor suppressors. RNA profiling of 314 colorectal adenomas/adenocarcinomas and 292 adjacent normal colon mucosa samples using RNA-sequencing demonstrated that the snoRNA host gene 16 (SNHG16) is significantly up-regulated in adenomas and all stages of CRC. SNHG16 expression was positively correlated to the expression of Wnt-regulated transcription factors, including ASCL2, ETS2, and c-Myc. In vitro abrogation of Wnt signaling in CRC cells reduced the expression of SNHG16 indicating that SNHG16 is regulated by the Wnt pathway. Silencing of SNHG16 resulted in reduced viability, increased apoptotic cell death and impaired cell migration. The SNHG16 silencing particularly affected expression of genes involved in lipid metabolism. A connection between SNHG16 and genes involved in lipid metabolism was also observed in clinical tumors. Argonaute CrossLinking and ImmunoPrecipitation (AGO-CLIP) demonstrated that SNHG16 heavily binds AGO and has 27 AGO/miRNA target sites along its length, indicating that SNHG16 may act as a competing endogenous RNA (ceRNA) "sponging" miRNAs off their cognate targets. Most interestingly, half of the miRNA families with high confidence targets on SNHG16 also target the 3'UTR of Stearoyl-CoA Desaturase (SCD). SCD is involved in lipid metabolism and is down-regulated upon SNHG16 silencing. In conclusion, up-regulation of SNHG16 is a frequent event in CRC, likely caused by deregulated Wnt signaling. In vitro analyses demonstrate that SNHG16 may play an oncogenic role in CRC and that it affects genes involved in lipid metabolism, possible through ceRNA related mechanisms.
Keywords: AGO-CLIP; Colorectal cancer; Functional analyses; Long non-coding RNAs; SNHG16; Wnt pathway.
Copyright © 2016 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures


References
-
- Atienza, J.M. , Yu, N. , Kirstein, S.L. , Xi, B. , Wang, X. , Xu, X. , Abassi, Y.A. , 2006. Dynamic and label-free cell-based assays using the real-time cell electronic sensing system. Assay. Drug Dev. Technol. 4, 597–607. - PubMed
-
- Bazzini, A.A. , Johnstone, T.G. , Christiano, R. , Mackowiak, S.D. , Obermayer, B. , Fleming, E.S. , Vejnar, C.E. , Lee, M.T. , Rajewsky, N. , Walther, T.C. , Giraldez, A.J. , 2014. Identification of small ORFs in vertebrates using ribosome footprinting and evolutionary conservation. EMBO J. 33, 981–993. - PMC - PubMed
-
- Birmingham, A. , Anderson, E.M. , Reynolds, A. , Ilsley-Tyree, D. , Leake, D. , Fedorov, Y. , Baskerville, S. , Maksimova, E. , Robinson, K. , Karpilow, J. , Marshall, W.S. , Khvorova, A. , 2006. 3′ UTR seed matches, but not overall identity, are associated with RNAi off-targets. Nat. Methods 3, 199–204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

