Ultra-High Resolution 3D Imaging of Whole Cells
- PMID: 27397506
- PMCID: PMC5005454
- DOI: 10.1016/j.cell.2016.06.016
Ultra-High Resolution 3D Imaging of Whole Cells
Abstract
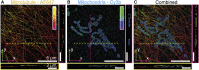
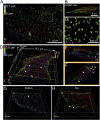
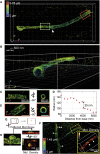
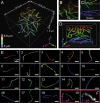
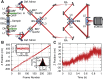
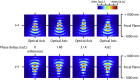
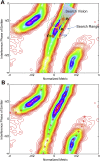
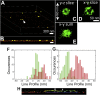
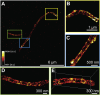
Fluorescence nanoscopy, or super-resolution microscopy, has become an important tool in cell biological research. However, because of its usually inferior resolution in the depth direction (50-80 nm) and rapidly deteriorating resolution in thick samples, its practical biological application has been effectively limited to two dimensions and thin samples. Here, we present the development of whole-cell 4Pi single-molecule switching nanoscopy (W-4PiSMSN), an optical nanoscope that allows imaging of three-dimensional (3D) structures at 10- to 20-nm resolution throughout entire mammalian cells. We demonstrate the wide applicability of W-4PiSMSN across diverse research fields by imaging complex molecular architectures ranging from bacteriophages to nuclear pores, cilia, and synaptonemal complexes in large 3D cellular volumes.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Aquino D., Schönle A., Geisler C., Middendorff C.V., Wurm C.A., Okamura Y., Lang T., Hell S.W., Egner A. Two-color nanoscopy of three-dimensional volumes by 4Pi detection of stochastically switched fluorophores. Nat. Methods. 2011;8:353–359. - PubMed
-
- Betzig E., Patterson G.H., Sougrat R., Lindwasser O.W., Olenych S., Bonifacino J.S., Davidson M.W., Lippincott-Schwartz J., Hess H.F. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313:1642–1645. - PubMed
-
- Bewersdorf J., Schmidt R., Hell S.W. Comparison of I5M and 4Pi-microscopy. J. Microsc. 2006;222:105–117. - PubMed
-
- Briggs J.A.G. Structural biology in situ--the potential of subtomogram averaging. Curr. Opin. Struct. Biol. 2013;23:261–267. - PubMed
Publication types
MeSH terms
Grants and funding
- 095927/A/11/Z/WT_/Wellcome Trust/United Kingdom
- P30 DK045735/DK/NIDDK NIH HHS/United States
- R21 HD078851/HD/NICHD NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- P01 GM099640/GM/NIGMS NIH HHS/United States
- S10 OD020142/OD/NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- C6946/A14492/CRUK_/Cancer Research UK/United Kingdom
- R01 HL124402/HL/NHLBI NIH HHS/United States
- 092096/WT_/Wellcome Trust/United Kingdom
- P30 CA034196/CA/NCI NIH HHS/United States
- R35 GM118084/GM/NIGMS NIH HHS/United States
- 203285/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- R01 GM105672/GM/NIGMS NIH HHS/United States
- R01 GM065835/GM/NIGMS NIH HHS/United States

