In vitro culture of ovarian follicles from Peromyscus
- PMID: 27397871
- PMCID: PMC5219864
- DOI: 10.1016/j.semcdb.2016.07.006
In vitro culture of ovarian follicles from Peromyscus
Abstract
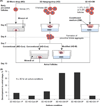
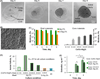
The ovarian follicle is the fundamental functional tissue unit of mammalian ovary. Each ovarian follicle contains one single oocyte. Isolation and in vitro culture of ovarian follicles to obtain fertilizable oocytes have been regarded as a promising strategy for women to combat infertility. The follicles from Peromyscus are considered as a better model than that from inbred mice for studying follicle culture. This is because Peromyscus mice are outbred (as with humans) with an increased life span. In this article, we reviewed studies on this subject conducted using Peromyscus follicles. These studies show that the conventional 2D micro-drop and 3D hanging-drop approaches established for in vitro culture of early preantral follicles from inbred mice are not directly applicable for cultivating the follicles from Peromyscus. However, the efficiency could be significantly improved by culturing multiple early preantral follicles in one hanging drop of Peromyscus ovarian cell-conditioned medium. It is further revealed that the mechanical heterogeneity in the extracellular matrix of ovary is crucial for developing early preantral follicles to the antral stage and for the subsequent ovulation to release cumulus-oocyte complex. These findings may provide valuable guidance for furthering the technology of in vitro follicle culture to restore fertility in the clinic.
Keywords: Alginate; Hanging drop; Mechanobiology; Microcapsule; Microfluidics; Ovulation.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures






References
-
- Andersen CY, Kristensen SG, Greve T, Schmidt KT. Cryopreservation of ovarian tissue for fertility preservation in young female oncological patients. Future Oncol. 2012;8:595–608. - PubMed
-
- Jin M, Yu Y, Huang H. An update on primary ovarian insufficiency. Sci China Life Sci. 2012;55:677–686. - PubMed
-
- Donnez J, Dolmans MM. Cryopreservation and transplantation of ovarian tissue. Clin Obstet Gynecol. 2010;53:787–796. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

