Photo-Triggered Click Chemistry for Biological Applications
- PMID: 27397964
- PMCID: PMC4935935
- DOI: 10.1007/s41061-015-0002-2
Photo-Triggered Click Chemistry for Biological Applications
Abstract
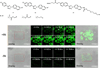
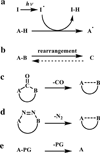
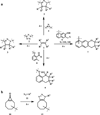
In the last decade and a half, numerous bioorthogonal reactions have been developed with a goal to study biological processes in their native environment, i.e., in living cells and animals. Among them, the photo-triggered reactions offer several unique advantages including operational simplicity with the use of light rather than toxic metal catalysts and ligands, and exceptional spatiotemporal control through the application of an appropriate light source with pre-selected wavelength, light intensity and exposure time. While the photoinduced reactions have been studied extensively in materials research, e.g., on macromolecular surface, the adaptation of these reactions for chemical biology applications is still in its infancy. In this chapter, we review the recent efforts in the discovery and optimization the photo-triggered bioorthogonal reactions, with a focus on those that have shown broad utility in biological systems. We discuss in each cases the chemical and mechanistic background, the kinetics of the reactions and the biological applicability together with the limiting factors.
Keywords: Azirine; Bioorthogonal reaction; Cyclopropenone; Hetero Diels–Alder reaction; Nitrile imine; Photo-triggered reaction; Photoclick; Tetrazole; o-Naphthoquinone methide; o-Quinodimethanes.
Figures






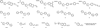



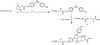

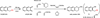



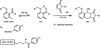
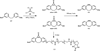
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
