Current use of routinely collected health data to complement randomized controlled trials: a meta-epidemiological survey
- PMID: 27398355
- PMCID: PMC4933635
- DOI: 10.9778/cmajo.20150036
Current use of routinely collected health data to complement randomized controlled trials: a meta-epidemiological survey
Abstract
Background: Studies that use routinely collected health data (RCD studies) are advocated to complement evidence from randomized controlled trials (RCTs) for comparative effectiveness research and to inform health care decisions when RCTs would be unfeasible. We aimed to evaluate the current use of routinely collected health data to complement RCT evidence.
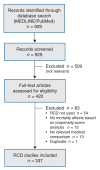
Methods: We searched PubMed for RCD studies published to 2010 that evaluated the comparative effectiveness of medical treatments on mortality using propensity scores. We identified RCTs of the same treatment comparisons and evaluated how frequently the RCD studies analyzed treatments that had not been compared previously in randomized trials. When RCTs did exist, we noted the claimed motivations for each RCD study. We also analyzed the citation impact of the RCD studies.
Results: Of 337 eligible RCD studies identified, 231 (68.5%) analyzed treatments that had already been compared in RCTs. The study investigators rarely claimed that it would be unethical (6/337) or difficult (18/337) to perform RCTs on the same question. Evidence from RCTs was mentioned or cited by authors of 213 RCD studies. The most common motivations for conducting the RCD studies were alleged limited generalizability of trial results to the "real world" (37.6%), evaluation of specific outcomes (31.9%) or specific populations (23.5%), and inconclusive or inconsistent evidence from randomized trials (25.8%). Studies evaluating "real world" effects had the lowest citation impact.
Interpretation: Most of the RCD studies we identified explored comparative treatment effects that had already been investigated in RCTs. The objective of such studies needs to shift more toward answering pivotal questions that are not supported by trial evidence or for which RCTs would be unfeasible.
Conflict of interest statement
Figures

References
-
- Spasoff RA. Epidemiologic methods for health policy. New York: Oxford University Press; 1999.
-
- Developing a protocol for observational comparative effectiveness research: a user's guide. Rockville (MD): Agency for Healthcare Research and Quality; 2013. - PubMed
-
- Cox E, Martin BC, Van Staa T, et al. Good research practices for comparative effectiveness research: approaches to mitigate bias and confounding in the design of nonrandomized studies of treatment effects using secondary data sources: the International Society for Pharmacoeconomics and Outcomes Research Good Research Practices for Retrospective Database Analysis Task Force Report-Part II. Value Health. 2009;12:1053–61. - PubMed
-
- Howie L, Hirsch B, Locklear T, et al. Assessing the value of patient-generated data to comparative effectiveness research. Health Aff (Millwood) 2014;33:1220–8. - PubMed
-
- Rassen JA, Schneeweiss S. Newly marketed medications present unique challenges for nonrandomized comparative effectiveness analyses. J Comp Eff Res. 2012;1:109–11. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
