Economic evaluation study (CHEER-compliant): Cost-effectiveness analysis of RAS screening for treatment of metastatic colorectal cancer based on the CALGB 80405 trial
- PMID: 27399059
- PMCID: PMC5058788
- DOI: 10.1097/MD.0000000000003762
Economic evaluation study (CHEER-compliant): Cost-effectiveness analysis of RAS screening for treatment of metastatic colorectal cancer based on the CALGB 80405 trial
Abstract
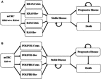
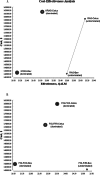
Cetuximab (Cetux)/Bevacizumab (Bev) treatments have shown considerably survival benefits for patients with metastatic colorectal cancer (mCRC) in the last decade. But they are costly. Currently, no data is available on the health economic implications of testing for extended RAS wild-type (wt) prior to Cetux/Bev treatments of patients with mCRC. This paper aimed to evaluate the cost-effectiveness of predictive testing for extended RAS-wt status in mCRC in the context of targeting the use of Cetux/Bev.Markov model 1 was conducted to provide evidence evaluating the cost-effectiveness of predictive testing for KRAS-wt or extended RAS-wt status based on treatments of chemotherapy plus Cetux/Bev. Markov model 2 assessed the cost-effectiveness of FOLFOX plus Cetux/Bev or FOLFIRI plus Cetux/Bev in extended RAS-wt population. Primary base case data were identified from the CALGB 80405 trial and the literatures. Costs were estimated from West China Hospital, Sichuan University, China. Survival benefits were reported in quality-adjusted life-years (QALYs). The incremental cost-effectiveness ratio (ICER) was calculated.In analysis 1, the cost per QALY was $88,394.09 for KRAS-Cetux, $80,797.82 for KRAS-Bev, $82,590.72 for RAS-Cetux, and $75,358.42 for RAS-Bev. The ICER for RAS-Cetux versus RAS-Bev was $420,700.50 per QALY gained. In analysis 2, the cost per QALY was $81,572.61, $80,856.50, $80,592.22, and $66,794.96 for FOLFOX-Cetux, FOLFOX-Bev, FOLFIRI-Cetux, and FOLFIRI-Bev, respectively. The analyses showed that the extended RAS-wt testing was less costly and more effective versus KRAS-wt testing before chemotherapy plus Cetux/Bev. Furthermore, FOLFIRI plus Bev was the most cost-effective strategy compared with others in extended RAS-wt population.It was economically favorable to identify patients with extended RAS-wt status. Furthermore, FOLFIRI plus Bev was the preferred strategy in extended RAS-wt patients.
Conflict of interest statement
The authors have no funding and conflicts of interest to disclose.
Figures

References
-
- Winder T, Lenz HJ. Vascular endothelial growth factor and epidermal growth factor signaling pathways as therapeutic targets for colorectal cancer. Gastroenterology 2010; 138:2163–2176. - PubMed
-
- Parks R, Gonen M, Kemeny N, et al. Adjuvant chemotherapy improves survival after resection of hepatic colorectal metastases: analysis of data from two continents. J Am Coll Surg 2007; 204:753–761. - PubMed
-
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003; 9:669–676. - PubMed
-
- Welch S, Spithoff K, Rumble RB, et al. Grp GCDS. Bevacizumab combined with chemotherapy for patients with advanced colorectal cancer: a systematic review. Ann Oncol 2010; 21:1152–1162. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

