Human islets contain four distinct subtypes of β cells
- PMID: 27399229
- PMCID: PMC4942571
- DOI: 10.1038/ncomms11756
Human islets contain four distinct subtypes of β cells
Abstract
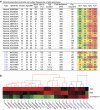
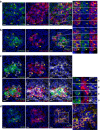
Human pancreatic islets of Langerhans contain five distinct endocrine cell types, each producing a characteristic hormone. The dysfunction or loss of the insulin-producing β cells causes diabetes mellitus, a disease that harms millions. Until now, β cells were generally regarded as a single, homogenous cell population. Here we identify four antigenically distinct subtypes of human β cells, which we refer to as β1-4, and which are distinguished by differential expression of ST8SIA1 and CD9. These subpopulations are always present in normal adult islets and have diverse gene expression profiles and distinct basal and glucose-stimulated insulin secretion. Importantly, the β cell subtype distribution is profoundly altered in type 2 diabetes. These data suggest that this antigenically defined β cell heterogeneity is functionally and likely medically relevant.
Conflict of interest statement
OHSU has commercially licensed some of the technology described herein (HPi2/HIC1-2B4, HPx1/HIC0-3B3, HPd3/DHIC5-4D9); C.D., P.R.S and M.G. are inventors of these antibodies. This potential conflict of interest has been reviewed and managed by OHSU. The remaining authors declare no competing financial interest.
Figures



References
-
- Lacy P. E. The pancreatic beta cell. Structure and function. N. Engl. J. Med. 276, 187–195 (1967). - PubMed
-
- Pipeleers D., Kiekens R., Ling Z., Wilikens A. & Schuit F. Physiologic relevance of heterogeneity in the pancreatic beta-cell population. Diabetologia 37, (Suppl 2): S57–S64 (1994). - PubMed
-
- Bernard-Kargar C., Kassis N., Berthault M. F., Pralong W. & Ktorza A. Sialylated form of the neural cell adhesion molecule (NCAM): a new tool for the identification and sorting of beta-cell subpopulations with different functional activity. Diabetes 50, (Suppl 1): S125–S130 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

