Genetic Architecture of Group A Streptococcal Necrotizing Soft Tissue Infections in the Mouse
- PMID: 27399650
- PMCID: PMC4939974
- DOI: 10.1371/journal.ppat.1005732
Genetic Architecture of Group A Streptococcal Necrotizing Soft Tissue Infections in the Mouse
Abstract
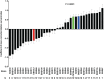
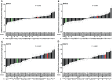
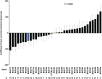
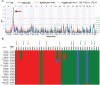
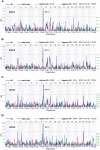
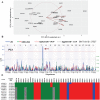
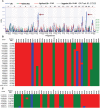
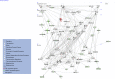
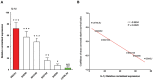
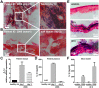
Host genetic variations play an important role in several pathogenic diseases, and we have previously provided strong evidences that these genetic variations contribute significantly to differences in susceptibility and clinical outcomes of invasive Group A Streptococcus (GAS) infections, including sepsis and necrotizing soft tissue infections (NSTIs). Our initial studies with conventional mouse strains revealed that host genetic variations and sex differences play an important role in orchestrating the severity, susceptibility and outcomes of NSTIs. To understand the complex genetic architecture of NSTIs, we utilized an unbiased, forward systems genetics approach in an advanced recombinant inbred (ARI) panel of mouse strains (BXD). Through this approach, we uncovered interactions between host genetics, and other non-genetic cofactors including sex, age and body weight in determining susceptibility to NSTIs. We mapped three NSTIs-associated phenotypic traits (i.e., survival, percent weight change, and lesion size) to underlying host genetic variations by using the WebQTL tool, and identified four NSTIs-associated quantitative genetic loci (QTL) for survival on mouse chromosome (Chr) 2, for weight change on Chr 7, and for lesion size on Chr 6 and 18 respectively. These QTL harbor several polymorphic genes. Identification of multiple QTL highlighted the complexity of the host-pathogen interactions involved in NSTI pathogenesis. We then analyzed and rank-ordered host candidate genes in these QTL by using the QTLminer tool and then developed a list of 375 candidate genes on the basis of annotation data and biological relevance to NSTIs. Further differential expression analyses revealed 125 genes to be significantly differentially regulated in susceptible strains compared to their uninfected controls. Several of these genes are involved in innate immunity, inflammatory response, cell growth, development and proliferation, and apoptosis. Additional network analyses using ingenuity pathway analysis (IPA) of these 125 genes revealed interleukin-1 beta network as key network involved in modulating the differential susceptibility to GAS NSTIs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Host Genetic Variations and Sex Differences Potentiate Predisposition, Severity, and Outcomes of Group A Streptococcus-Mediated Necrotizing Soft Tissue Infections.Infect Immun. 2015 Nov 16;84(2):416-24. doi: 10.1128/IAI.01191-15. Print 2016 Feb. Infect Immun. 2015. PMID: 26573737 Free PMC article.
-
Systems Genetics Approaches in Mouse Models of Group A Streptococcal Necrotizing Soft-Tissue Infections.Adv Exp Med Biol. 2020;1294:151-166. doi: 10.1007/978-3-030-57616-5_10. Adv Exp Med Biol. 2020. PMID: 33079368 Review.
-
An unbiased systems genetics approach to mapping genetic loci modulating susceptibility to severe streptococcal sepsis.PLoS Pathog. 2008 Apr 18;4(4):e1000042. doi: 10.1371/journal.ppat.1000042. PLoS Pathog. 2008. PMID: 18421376 Free PMC article.
-
Pathogenic Mechanisms of Streptococcal Necrotizing Soft Tissue Infections.Adv Exp Med Biol. 2020;1294:127-150. doi: 10.1007/978-3-030-57616-5_9. Adv Exp Med Biol. 2020. PMID: 33079367 Review.
-
Susceptibility to severe Streptococcal sepsis: use of a large set of isogenic mouse lines to study genetic and environmental factors.Genes Immun. 2007 Jul;8(5):404-15. doi: 10.1038/sj.gene.6364402. Epub 2007 May 24. Genes Immun. 2007. PMID: 17525705
Cited by
-
Serial irrigation and debridement with the use of Kerecis in the treatment of necrotizing fasciitis: A case report.Trauma Case Rep. 2025 May 27;58:101196. doi: 10.1016/j.tcr.2025.101196. eCollection 2025 Aug. Trauma Case Rep. 2025. PMID: 40519275 Free PMC article.
-
Reduced interleukin-18 secretion by human monocytic cells in response to infections with hyper-virulent Streptococcus pyogenes.J Biomed Sci. 2024 Feb 27;31(1):26. doi: 10.1186/s12929-024-01014-9. J Biomed Sci. 2024. PMID: 38408992 Free PMC article.
-
Concurrent Brain Subregion Microgliosis in an HLA-II Mouse Model of Group A Streptococcal Skin Infection.Microorganisms. 2023 Sep 20;11(9):2356. doi: 10.3390/microorganisms11092356. Microorganisms. 2023. PMID: 37764200 Free PMC article.
-
Inhibition of Inflammasome-Dependent Interleukin 1β Production by Streptococcal NAD+-Glycohydrolase: Evidence for Extracellular Activity.mBio. 2017 Jul 18;8(4):e00756-17. doi: 10.1128/mBio.00756-17. mBio. 2017. PMID: 28720729 Free PMC article.
-
The Secreted Virulence Factor NADase of Group A Streptococcus Inhibits P2X7 Receptor-Mediated Release of IL-1β.Front Immunol. 2019 Jun 18;10:1385. doi: 10.3389/fimmu.2019.01385. eCollection 2019. Front Immunol. 2019. PMID: 31275321 Free PMC article.
References
-
- Demers B, Simor AE, Vellend H, Schlievert PM, Byrne S, Jamieson F, et al. Severe invasive group A streptococcal infections in Ontario, Canada: 1987–1991. Clin Infect Dis. 1993;16(6):792–800; discussion 1–2. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

