A PDMS-Based Microfluidic Hanging Drop Chip for Embryoid Body Formation
- PMID: 27399655
- PMCID: PMC6272923
- DOI: 10.3390/molecules21070882
A PDMS-Based Microfluidic Hanging Drop Chip for Embryoid Body Formation
Abstract
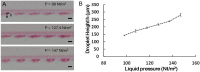
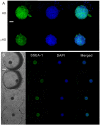
The conventional hanging drop technique is the most widely used method for embryoid body (EB) formation. However, this method is labor intensive and limited by the difficulty in exchanging the medium. Here, we report a microfluidic chip-based approach for high-throughput formation of EBs. The device consists of microfluidic channels with 6 × 12 opening wells in PDMS supported by a glass substrate. The PDMS channels were fabricated by replicating polydimethyl-siloxane (PDMS) from SU-8 mold. The droplet formation in the chip was tested with different hydrostatic pressures to obtain optimal operation pressures for the wells with 1000 μm diameter openings. The droplets formed at the opening wells were used to culture mouse embryonic stem cells which could subsequently developed into EBs in the hanging droplets. This device also allows for medium exchange of the hanging droplets making it possible to perform immunochemistry staining and characterize EBs on chip.
Keywords: embryoid body; embryonic stem cell; microfluidic hanging drop.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Effects of hanging drop culture conditions on embryoid body formation and neuronal cell differentiation using mouse embryonic stem cells: optimization of culture conditions for the formation of well-controlled embryoid bodies.J Biosci Bioeng. 2013 May;115(5):571-4. doi: 10.1016/j.jbiosc.2012.11.016. Epub 2012 Dec 29. J Biosci Bioeng. 2013. PMID: 23276518
-
Effect of separation distance on the growth and differentiation of mouse embryoid bodies in micropatterned cultures.J Biosci Bioeng. 2016 Jan;121(1):105-110. doi: 10.1016/j.jbiosc.2015.04.018. Epub 2015 Jun 2. J Biosci Bioeng. 2016. PMID: 26047736
-
Embryoid body culture of mouse embryonic stem cells using microwell and micropatterned chips.J Biosci Bioeng. 2011 Jan;111(1):85-91. doi: 10.1016/j.jbiosc.2010.08.014. Epub 2010 Sep 21. J Biosci Bioeng. 2011. PMID: 20863754
-
Over a century of neuron culture: from the hanging drop to microfluidic devices.Yale J Biol Med. 2012 Dec;85(4):501-21. Epub 2012 Dec 13. Yale J Biol Med. 2012. PMID: 23239951 Free PMC article. Review.
-
Circuit-Based Design of Microfluidic Drop Networks.Micromachines (Basel). 2022 Jul 16;13(7):1124. doi: 10.3390/mi13071124. Micromachines (Basel). 2022. PMID: 35888941 Free PMC article. Review.
Cited by
-
Three-Dimensional In Vitro Cell Culture Models for Efficient Drug Discovery: Progress So Far and Future Prospects.Pharmaceuticals (Basel). 2022 Jul 27;15(8):926. doi: 10.3390/ph15080926. Pharmaceuticals (Basel). 2022. PMID: 36015074 Free PMC article. Review.
-
3D culture applied to reproduction in females: possibilities and perspectives.Anim Reprod. 2024 Mar 8;21(1):e20230039. doi: 10.1590/1984-3143-AR2023-0039. eCollection 2024. Anim Reprod. 2024. PMID: 38510565 Free PMC article. Review.
-
Emerging brain organoids: 3D models to decipher, identify and revolutionize brain.Bioact Mater. 2025 Feb 12;47:378-402. doi: 10.1016/j.bioactmat.2025.01.025. eCollection 2025 May. Bioact Mater. 2025. PMID: 40026825 Free PMC article. Review.
-
Three-Dimensional Spheroids for Cancer Research.Methods Mol Biol. 2023;2645:65-103. doi: 10.1007/978-1-0716-3056-3_3. Methods Mol Biol. 2023. PMID: 37202612
-
Spheroid Engineering in Microfluidic Devices.ACS Omega. 2023 Jan 18;8(4):3630-3649. doi: 10.1021/acsomega.2c06052. eCollection 2023 Jan 31. ACS Omega. 2023. PMID: 36743071 Free PMC article. Review.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

