Citrullination of histone H3 drives IL-6 production by bone marrow mesenchymal stem cells in MGUS and multiple myeloma
- PMID: 27400413
- PMCID: PMC5292682
- DOI: 10.1038/leu.2016.187
Citrullination of histone H3 drives IL-6 production by bone marrow mesenchymal stem cells in MGUS and multiple myeloma
Abstract
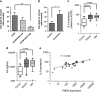
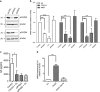
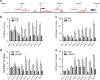
Multiple myeloma (MM), an incurable plasma cell malignancy, requires localisation within the bone marrow. This microenvironment facilitates crucial interactions between the cancer cells and stromal cell types that permit the tumour to survive and proliferate. There is increasing evidence that the bone marrow mesenchymal stem cell (BMMSC) is stably altered in patients with MM-a phenotype also postulated to exist in patients with monoclonal gammopathy of undetermined significance (MGUS) a benign condition that precedes MM. In this study, we describe a mechanism by which increased expression of peptidyl arginine deiminase 2 (PADI2) by BMMSCs in patients with MGUS and MM directly alters malignant plasma cell phenotype. We identify PADI2 as one of the most highly upregulated transcripts in BMMSCs from both MGUS and MM patients, and that through its enzymatic deimination of histone H3 arginine 26, PADI2 activity directly induces the upregulation of interleukin-6 expression. This leads to the acquisition of resistance to the chemotherapeutic agent, bortezomib, by malignant plasma cells. We therefore describe a novel mechanism by which BMMSC dysfunction in patients with MGUS and MM directly leads to pro-malignancy signalling through the citrullination of histone H3R26.
Figures



Similar articles
-
Bone marrow fibroblasts overexpress miR-27b and miR-214 in step with multiple myeloma progression, dependent on tumour cell-derived exosomes.J Pathol. 2019 Feb;247(2):241-253. doi: 10.1002/path.5187. J Pathol. 2019. PMID: 30357841
-
Upregulation of Syndecan-1 in the bone marrow microenvironment in multiple myeloma is associated with angiogenesis.Eur J Haematol. 2015 Sep;95(3):211-7. doi: 10.1111/ejh.12473. Epub 2015 Jan 7. Eur J Haematol. 2015. PMID: 25353275
-
Interleukin-6 is expressed by plasma cells from patients with multiple myeloma and monoclonal gammopathy of undetermined significance.Br J Haematol. 1998 May;101(2):287-95. doi: 10.1046/j.1365-2141.1998.00687.x. Br J Haematol. 1998. PMID: 9609524
-
Contributions of the Bone Microenvironment to Monoclonal Gammopathy of Undetermined Significance Pathogenesis.Curr Osteoporos Rep. 2018 Dec;16(6):635-641. doi: 10.1007/s11914-018-0479-z. Curr Osteoporos Rep. 2018. PMID: 30229522 Free PMC article. Review.
-
Monoclonal gammopathy of undetermined significance and smoldering multiple myeloma.Hematol Oncol Clin North Am. 2014 Oct;28(5):775-90. doi: 10.1016/j.hoc.2014.06.005. Epub 2014 Jul 22. Hematol Oncol Clin North Am. 2014. PMID: 25212882 Review.
Cited by
-
Network-based analysis of the molecular mechanisms of multiple myeloma and monoclonal gammopathy of undetermined significance.Oncol Lett. 2017 Oct;14(4):4167-4175. doi: 10.3892/ol.2017.6723. Epub 2017 Aug 4. Oncol Lett. 2017. PMID: 28943924 Free PMC article.
-
A Novel Citrullinated Modification of Histone 3 and Its Regulatory Mechanisms Related to IPO-38 Antibody-Labeled Protein.Front Oncol. 2019 Apr 18;9:304. doi: 10.3389/fonc.2019.00304. eCollection 2019. Front Oncol. 2019. PMID: 31058095 Free PMC article.
-
Putative Roles for Peptidylarginine Deiminases in COVID-19.Int J Mol Sci. 2020 Jun 30;21(13):4662. doi: 10.3390/ijms21134662. Int J Mol Sci. 2020. PMID: 32629995 Free PMC article.
-
Histone citrullination: a new target for tumors.Mol Cancer. 2021 Jun 11;20(1):90. doi: 10.1186/s12943-021-01373-z. Mol Cancer. 2021. PMID: 34116679 Free PMC article. Review.
-
Peptidylarginine Deiminases Post-Translationally Deiminate Prohibitin and Modulate Extracellular Vesicle Release and MicroRNAs in Glioblastoma Multiforme.Int J Mol Sci. 2018 Dec 28;20(1):103. doi: 10.3390/ijms20010103. Int J Mol Sci. 2018. PMID: 30597867 Free PMC article.
References
-
- Landgren O. Monoclonal gammopathy of undetermined significance and smoldering multiple myeloma: biological insights and early treatment strategies. Hematology 2013; 2013: 478–487. - PubMed
-
- Hayashi T, Hideshima T, Akiyama M, Richardson P, Schlossman RL, Chauhan D et al. Arsenic trioxide inhibits growth of human multiple myeloma cells in the bone marrow microenvironment. Mol Cancer Ther 2002; 1: 851–860. - PubMed
-
- Mitsiades CS, McMillin DW, Klippel S, Hideshima T, Chauhan D, Richardson PG et al. The role of the bone marrow microenvironment in the pathophysiology of myeloma and its significance in the development of more effective therapies. Hematol Oncol Clin North Am 2007; 21: 1007–1034, vii-viii. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

