Platelet-Rich Plasma Activates Proinflammatory Signaling Pathways and Induces Oxidative Stress in Tendon Fibroblasts
- PMID: 27400714
- PMCID: PMC4970921
- DOI: 10.1177/0363546516637176
Platelet-Rich Plasma Activates Proinflammatory Signaling Pathways and Induces Oxidative Stress in Tendon Fibroblasts
Abstract
Background: Tendon injuries are one of the most common musculoskeletal conditions in active patients. Platelet-rich plasma (PRP) has shown some promise in the treatment of tendon disorders, but little is known as to the mechanisms by which PRP can improve tendon regeneration. PRP contains numerous different growth factors and cytokines that activate various cellular signaling cascades, but it has been difficult to determine precisely which signaling pathways and cellular responses are activated after PRP treatment. Additionally, macrophages play an important role in modulating tendon regeneration, but the influence of PRP on determining whether macrophages assume a proinflammatory or anti-inflammatory phenotype remains unknown.
Purpose: To use genome-wide expression profiling, bioinformatics, and protein analysis to determine the cellular pathways activated in fibroblasts treated with PRP. The effect of PRP on macrophage polarization was also evaluated.
Study design: Controlled laboratory study.
Methods: Tendon fibroblasts or macrophages from rats were cultured and treated with either platelet-poor plasma (PPP) or PRP. RNA or protein was isolated from cells and analyzed using microarrays, quantitative polymerase chain reaction, immunoblotting, or bioinformatics techniques.
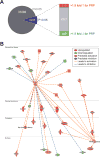
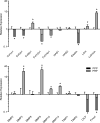
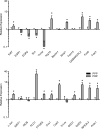
Results: Pathway analysis determined that the most highly induced signaling pathways in PRP-treated tendon fibroblasts were TNFα and NFκB pathways. PRP also downregulated the expression of extracellular matrix genes and induced the expression of autophagy-related genes and reactive oxygen species (ROS) genes and protein markers in tendon fibroblasts. PRP failed to have a major effect on markers of macrophage polarization.
Conclusion: PRP induces an inflammatory response in tendon fibroblasts, which leads to the formation of ROS and the activation of oxidative stress pathways. PRP does not appear to significantly modulate macrophage polarization.
Clinical relevance: PRP might act by inducing a transient inflammatory event, which could then trigger a tissue regeneration response.
Keywords: autophagy; inflammation; oxidative stress; platelet-rich plasma; tendinopathy; tendon.
© 2016 The Author(s).
Figures
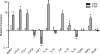


Similar articles
-
Harmful Effects of Leukocyte-Rich Platelet-Rich Plasma on Rabbit Tendon Stem Cells In Vitro.Am J Sports Med. 2016 Aug;44(8):1941-51. doi: 10.1177/0363546516644718. Epub 2016 May 16. Am J Sports Med. 2016. PMID: 27184544
-
Platelet-rich plasma stimulates cell proliferation and enhances matrix gene expression and synthesis in tenocytes from human rotator cuff tendons with degenerative tears.Am J Sports Med. 2012 May;40(5):1035-45. doi: 10.1177/0363546512437525. Epub 2012 Feb 23. Am J Sports Med. 2012. PMID: 22366517
-
Tendon derived stem cells promote platelet-rich plasma healing in collagenase-induced rat achilles tendinopathy.Cell Physiol Biochem. 2014;34(6):2153-68. doi: 10.1159/000369659. Epub 2014 Dec 2. Cell Physiol Biochem. 2014. PMID: 25562162
-
Profound Properties of Protein-Rich, Platelet-Rich Plasma Matrices as Novel, Multi-Purpose Biological Platforms in Tissue Repair, Regeneration, and Wound Healing.Int J Mol Sci. 2024 Jul 19;25(14):7914. doi: 10.3390/ijms25147914. Int J Mol Sci. 2024. PMID: 39063156 Free PMC article. Review.
-
Advances with platelet rich plasma therapies for tendon regeneration.Expert Opin Biol Ther. 2018 Apr;18(4):389-398. doi: 10.1080/14712598.2018.1424626. Epub 2018 Jan 7. Expert Opin Biol Ther. 2018. PMID: 29300106 Review.
Cited by
-
The Biological Effect of Platelet-Rich Plasma on Rotator Cuff Tears: A Prospective Randomized In Vivo Study.Int J Mol Sci. 2024 Jul 21;25(14):7957. doi: 10.3390/ijms25147957. Int J Mol Sci. 2024. PMID: 39063199 Free PMC article. Clinical Trial.
-
Therapeutic Potential of Platelet-Rich Plasma in Fracture Healing: A Comprehensive Review.Cureus. 2024 Jun 12;16(6):e62271. doi: 10.7759/cureus.62271. eCollection 2024 Jun. Cureus. 2024. PMID: 39006629 Free PMC article. Review.
-
Bedside to bench and back to bedside: Translational implications of targeted intervertebral disc therapeutics.J Orthop Translat. 2017 Apr 21;10:18-27. doi: 10.1016/j.jot.2017.03.008. eCollection 2017 Jul. J Orthop Translat. 2017. PMID: 29662757 Free PMC article. Review.
-
Molecular Insights into the Superiority of Platelet Lysate over FBS for hASC Expansion and Wound Healing.Cells. 2025 Jul 25;14(15):1154. doi: 10.3390/cells14151154. Cells. 2025. PMID: 40801587 Free PMC article.
-
In vitro responses to platelet-rich-plasma are associated with variable clinical outcomes in patients with knee osteoarthritis.Sci Rep. 2021 Jun 1;11(1):11493. doi: 10.1038/s41598-021-90174-x. Sci Rep. 2021. PMID: 34075069 Free PMC article. Clinical Trial.
References
-
- Castillo TN, Pouliot MA, Kim H-J, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39(2):266–271. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

