YPEL3 suppresses epithelial-mesenchymal transition and metastasis of nasopharyngeal carcinoma cells through the Wnt/β-catenin signaling pathway
- PMID: 27400785
- PMCID: PMC4940860
- DOI: 10.1186/s13046-016-0384-1
YPEL3 suppresses epithelial-mesenchymal transition and metastasis of nasopharyngeal carcinoma cells through the Wnt/β-catenin signaling pathway
Erratum in
-
Correction to: YPEL3 suppresses epithelial-mesenchymal transition and metastasis of nasopharyngeal carcinoma cells through the Wnt/β-catenin signaling pathway.J Exp Clin Cancer Res. 2020 Oct 12;39(1):214. doi: 10.1186/s13046-020-01710-y. J Exp Clin Cancer Res. 2020. PMID: 33046124 Free PMC article.
-
Correction to: YPEL3 suppresses epithelial-mesenchymal transition and metastasis of nasopharyngeal carcinoma cells through the Wnt/β-catenin signaling pathway.J Exp Clin Cancer Res. 2021 Dec 23;40(1):400. doi: 10.1186/s13046-021-02189-x. J Exp Clin Cancer Res. 2021. PMID: 34949224 Free PMC article. No abstract available.
Abstract
Background: Metastasis remains the major cause of death in nasopharyngeal carcinoma (NPC). Yippee-like 3 (YPEL3) plays an important role in tumorigenesis. However, its function and mechanism in NPC has not been systematically explored.
Methods: We evaluated YPEL3 expression in NPC cell lines and tissues using real-time PCR and western blotting. Then, we established NPC cell lines that stably overexpressed YPEL3 and knocked down YPEL3 expression to explore its function in NPC in vitro and in vivo. Additionally, we investigated the potential mechanism of YPEL3 action by identifying the Wnt/β-catenin signaling pathway downstream genes using western blotting.
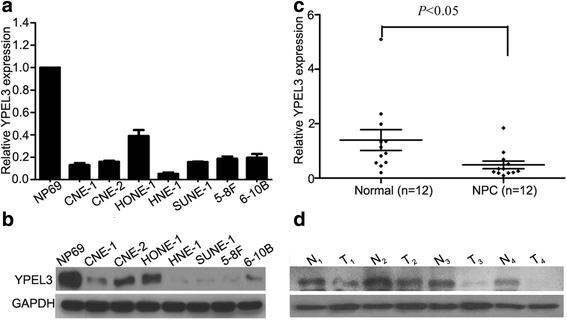
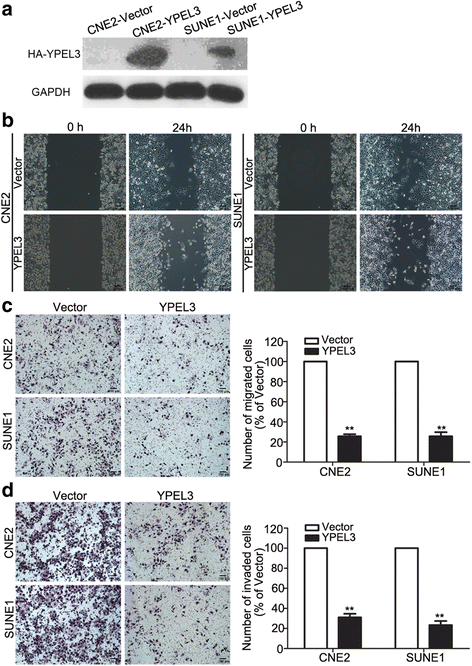
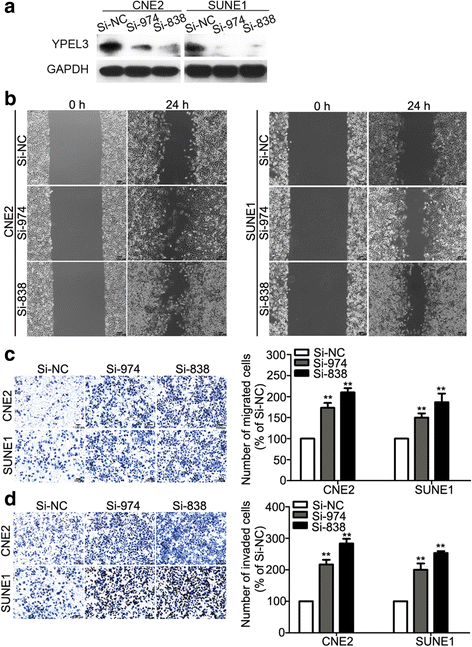
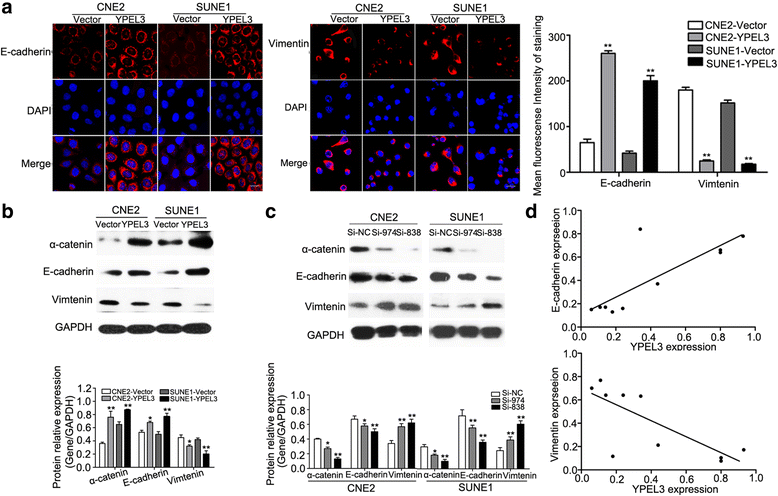
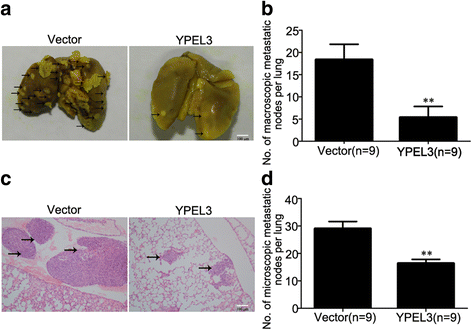
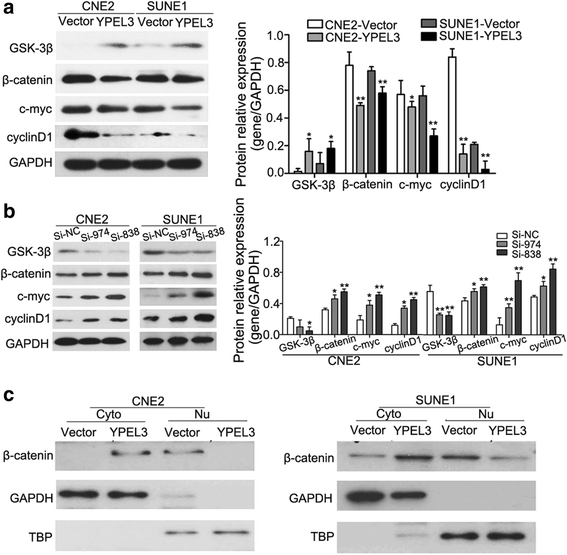
Results: YPEL3 was downregulated in NPC cell lines and tissue samples. Ectopic expression of YPEL3 inhibited NPC cell migration and invasion in vitro; while silencing of YPEL3 promoted NPC cell migration and invasion. Further study indicated that overexpression of YPEL3 inhibited NPC cell epithelial-mesenchymal transition (EMT) and that silencing it enhanced EMT. Overexpression of YPEL3 suppressed NPC cell lung metastasis in vivo. The mechanism study determined that YPEL3 suppressed the expression levels of Wnt/β-catenin signaling pathway downstream genes and the nuclear translocation of β-catenin.
Conclusions: YPEL3 suppresses NPC EMT and metastasis by suppressing the Wnt/β-catenin signaling pathway, which would help better understanding the molecular mechanisms of NPC metastasis and provide novel therapeutic targets for NPC treatment.
Keywords: Epithelial–mesenchymal transition; Metastasis; Nasopharyngeal carcinoma; Wnt/β-catenin; YPEL3.
Figures
Similar articles
-
MicroRNA-506 inhibits tumor growth and metastasis in nasopharyngeal carcinoma through the inactivation of the Wnt/β-catenin signaling pathway by down-regulating LHX2.J Exp Clin Cancer Res. 2019 Feb 21;38(1):97. doi: 10.1186/s13046-019-1023-4. J Exp Clin Cancer Res. 2019. Retraction in: J Exp Clin Cancer Res. 2021 Sep 30;40(1):309. doi: 10.1186/s13046-021-02113-3. PMID: 30791932 Free PMC article. Retracted. Clinical Trial.
-
ZNF488 Enhances the Invasion and Tumorigenesis in Nasopharyngeal Carcinoma Via the Wnt Signaling Pathway Involving Epithelial Mesenchymal Transition.Cancer Res Treat. 2016 Jan;48(1):334-44. doi: 10.4143/crt.2014.311. Epub 2015 Mar 12. Cancer Res Treat. 2016. PMID: 25779368 Free PMC article.
-
Protocadherin20 Acts as a Tumor Suppressor Gene: Epigenetic Inactivation in Nasopharyngeal Carcinoma.J Cell Biochem. 2015 Aug;116(8):1766-75. doi: 10.1002/jcb.25135. J Cell Biochem. 2015. PMID: 25736877
-
Long non-coding RNAs as the pivotal regulators of epithelial mesenchymal transition through WNT/β-catenin signaling pathway in tumor cells.Pathol Res Pract. 2024 Nov;263:155683. doi: 10.1016/j.prp.2024.155683. Epub 2024 Oct 28. Pathol Res Pract. 2024. PMID: 39471528 Review.
-
Wnt/β-catenin-driven EMT regulation in human cancers.Cell Mol Life Sci. 2024 Feb 9;81(1):79. doi: 10.1007/s00018-023-05099-7. Cell Mol Life Sci. 2024. PMID: 38334836 Free PMC article. Review.
Cited by
-
Loss of IRF2BPL impairs neuronal maintenance through excess Wnt signaling.Sci Adv. 2022 Jan 21;8(3):eabl5613. doi: 10.1126/sciadv.abl5613. Epub 2022 Jan 19. Sci Adv. 2022. PMID: 35044823 Free PMC article.
-
DDX21 functions as a potential novel oncopromoter in pancreatic ductal adenocarcinoma: a comprehensive analysis of the DExD box family.Discov Oncol. 2024 Aug 3;15(1):333. doi: 10.1007/s12672-024-01204-9. Discov Oncol. 2024. PMID: 39095628 Free PMC article.
-
Kindlin-2 promotes hepatocellular carcinoma invasion and metastasis by increasing Wnt/β-catenin signaling.J Exp Clin Cancer Res. 2017 Sep 29;36(1):134. doi: 10.1186/s13046-017-0603-4. J Exp Clin Cancer Res. 2017. Retraction in: J Exp Clin Cancer Res. 2025 Feb 28;44(1):76. doi: 10.1186/s13046-025-03333-7. PMID: 28969700 Free PMC article. Retracted.
-
TIPE2 Inhibits the Expression of Asthma-Related Inflammatory Factors in Hyperstretched Bronchial Epithelial Cells Through the Wnt/β-Catenin Pathway.Inflammation. 2017 Jun;40(3):770-777. doi: 10.1007/s10753-017-0521-9. Inflammation. 2017. PMID: 28188409
-
Overexpression of ANXA3 is an independent prognostic indicator in gastric cancer and its depletion suppresses cell proliferation and tumor growth.Oncotarget. 2016 Dec 27;7(52):86972-86984. doi: 10.18632/oncotarget.13493. Oncotarget. 2016. PMID: 27894078 Free PMC article.
References
-
- Lai SZ, Li WF, Chen L, Luo W, Chen YY, Liu LZ, et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol, Biol, Phys. 2011;80(3):661–668. doi: 10.1016/j.ijrobp.2010.03.024. - DOI - PubMed
-
- Chen YP, Wang ZX, Chen L, Liu X, Tang LL, Mao YP, et al. A Bayesian network meta-analysis comparing concurrent chemoradiotherapy followed by adjuvant chemotherapy, concurrent chemoradiotherapy alone and radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. Ann Oncol. 2015;26(1):205–211. doi: 10.1093/annonc/mdu507. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

