Externally Applied Static Magnetic Field Enhances Cardiac Retention and Functional Benefit of Magnetically Iron-Labeled Adipose-Derived Stem Cells in Infarcted Hearts
- PMID: 27400797
- PMCID: PMC5031175
- DOI: 10.5966/sctm.2015-0220
Externally Applied Static Magnetic Field Enhances Cardiac Retention and Functional Benefit of Magnetically Iron-Labeled Adipose-Derived Stem Cells in Infarcted Hearts
Abstract
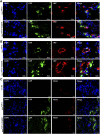
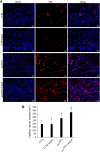
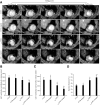
: Although adipose-derived stem cells (ASCs) hold the promise of effective therapy for myocardial infarction, low cardiac retention of implanted ASCs has hindered their therapeutic efficiency. We investigated whether an externally applied static magnetic field (SMF) enhances cardiac localization of "magnetic" cells and promotes heart function recovery when ASCs are preloaded with superparamagnetic iron oxide (SPIO) nanoparticles. The influence of SMF (0.1 Tesla) on the biological activities of SPIO-labeled ASCs (SPIOASCs) was investigated first. Fifty-six female rats with myocardial infarction underwent intramyocardial injection of cell culture medium (CCM) or male SPIOASCs with or without the subcutaneous implantable magnet (CCM-magnet or SPIOASC-magnet). Four weeks later, endothelial differentiation, angiogenic cytokine secretion, angiogenesis, cardiomyocyte apoptosis, cell retention, and cardiac performance were examined. The 0.1-Tsela SMF did not adversely affect the viability, proliferation, angiogenic cytokine secretion, and DNA integrity of SPIOASCs. The implanted SPIOASCs could differentiate into endothelial cell, incorporate into newly formed vessels, and secrete multiple angiogenic cytokines. Four weeks after cell transplantation, the number of cardiac SPIOASCs was significantly increased, vascular density was markedly enlarged, fewer apoptotic cardiomyocytes were present, and heart contractile function was substantially improved in the SPIOASC-magnet treated rats in comparison with the SPIOASC-treated rats. The SPIOASCs could differentiate into endothelial cells, incorporate into vessels, promote angiogenesis, and inhibit ischemic cardiomyocyte apoptosis. An externally applied SMF offered a secure environment for biological properties of SPIOASCs, increased the cardiac retention of implanted magnetic SPIOASCs, and further enhanced heart function recovery after myocardial infarction.
Significance: This pilot proof-of-concept study suggests that a 0.1-Tesla static magnetic field does not adversely affect the viability, proliferation, angiogenic cytokine secretion, or DNA integrity of the superparamagnetic iron oxide-labeled adipose-derived stem cells (SPIOASCs). Implantation of adipose-derived stem cells promotes myocardial neovascularization and inhibits ischemic cardiomyocyte apoptosis through endothelial differentiation, incorporation into vessels, and paracrine factor secretion. An externally applied static magnetic field enhanced myocardial retention of intramyocardially injected "magnetic" SPIOASCs and promoted cardiac function recovery after myocardial infarction. With further preclinical optimization, this approach may improve the outcome of current stem cell therapy for ischemic myocardial infarction.
Keywords: Adipose-derived stem cells; Cell retention; Myocardial infarction; Static magnetic field; Superparamagnetic iron oxide.
©AlphaMed Press.
Figures





Similar articles
-
Hypoxia enhances the therapeutic potential of superparamagnetic iron oxide-labeled adipose-derived stem cells for myocardial infarction.J Huazhong Univ Sci Technolog Med Sci. 2017 Aug;37(4):516-522. doi: 10.1007/s11596-017-1766-0. Epub 2017 Aug 8. J Huazhong Univ Sci Technolog Med Sci. 2017. PMID: 28786062
-
Adipose-derived stem cells from both visceral and subcutaneous fat deposits significantly improve contractile function of infarcted rat hearts.Cell Transplant. 2015;24(11):2337-51. doi: 10.3727/096368914X685780. Epub 2015 Jan 5. Cell Transplant. 2015. PMID: 25562327
-
Superparamagnetic iron oxide does not affect the viability and function of adipose-derived stem cells, and superparamagnetic iron oxide-enhanced magnetic resonance imaging identifies viable cells.Magn Reson Imaging. 2009 Jan;27(1):108-19. doi: 10.1016/j.mri.2008.05.013. Epub 2008 Jul 26. Magn Reson Imaging. 2009. PMID: 18657922
-
Myocardial regeneration potential of adipose tissue-derived stem cells.Biochem Biophys Res Commun. 2010 Oct 22;401(3):321-6. doi: 10.1016/j.bbrc.2010.09.012. Epub 2010 Sep 15. Biochem Biophys Res Commun. 2010. PMID: 20833143 Review.
-
Harnessing the secretome of adipose-derived stem cells in the treatment of ischemic heart diseases.Stem Cell Res Ther. 2019 Jun 27;10(1):196. doi: 10.1186/s13287-019-1289-7. Stem Cell Res Ther. 2019. PMID: 31248452 Free PMC article. Review.
Cited by
-
Overexpression of integrin β2 improves migration and engraftment of adipose-derived stem cells and augments angiogenesis in myocardial infarction.Ann Transl Med. 2022 Aug;10(16):863. doi: 10.21037/atm-22-3339. Ann Transl Med. 2022. PMID: 36111003 Free PMC article.
-
Stem Cell Therapy in Ischemic Heart Failure.Am J Cardiovasc Drugs. 2025 Sep;25(5):601-632. doi: 10.1007/s40256-025-00741-0. Epub 2025 Jun 27. Am J Cardiovasc Drugs. 2025. PMID: 40576736 Review.
-
Magnetically Assisted Control of Stem Cells Applied in 2D, 3D and In Situ Models of Cell Migration.Molecules. 2019 Apr 19;24(8):1563. doi: 10.3390/molecules24081563. Molecules. 2019. PMID: 31010261 Free PMC article.
-
Magnetic Field Promotes Migration of Schwann Cells with Chondroitinase ABC (ChABC)-Loaded Superparamagnetic Nanoparticles Across Astrocyte Boundary in vitro.Int J Nanomedicine. 2020 Jan 20;15:315-332. doi: 10.2147/IJN.S227328. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32021182 Free PMC article.
-
Application of adipose-derived stem cells in ischemic heart disease: theory, potency, and advantage.Front Cardiovasc Med. 2024 Jan 19;11:1324447. doi: 10.3389/fcvm.2024.1324447. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38312236 Free PMC article. Review.
References
-
- Planat-Benard V, Silvestre JS, Cousin B, et al. Plasticity of human adipose lineage cells toward endothelial cells: Physiological and therapeutic perspectives. Circulation. 2004;109:656–663. - PubMed
-
- Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. - PubMed
-
- De Ugarte DA, Morizono K, Elbarbary A, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

