Outcome of Patients With Recurrent Osteosarcoma Enrolled in Seven Phase II Trials Through Children's Cancer Group, Pediatric Oncology Group, and Children's Oncology Group: Learning From the Past to Move Forward
- PMID: 27400942
- PMCID: PMC5012712
- DOI: 10.1200/JCO.2015.65.5381
Outcome of Patients With Recurrent Osteosarcoma Enrolled in Seven Phase II Trials Through Children's Cancer Group, Pediatric Oncology Group, and Children's Oncology Group: Learning From the Past to Move Forward
Abstract
Purpose: The use of radiographic response as the primary end point in phase II osteosarcoma trials may limit optimal detection of treatment response because of the calcified tumor matrix. We performed this study to determine if time to progression could be used as an end point for subsequent studies.
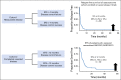
Patients and methods: We performed a retrospective analysis of outcome for patients with recurrent/refractory osteosarcoma enrolled in one of seven phase II trials conducted by the Children's Oncology Group and predecessor groups from 1997 to 2007. All trials used RECIST or WHO radiographic response criteria and the primary end point of response rate. The following potential prognostic factors-age, trial, number of prior chemotherapy regimens, sex, and race/ethnicity-were evaluated for their impact on event-free survival (EFS). We used data from a phase II study (AOST0221) of patients with osteosarcoma who were given inhaled granulocyte-macrophage colony-stimulating factor with first pulmonary recurrence who had an EFS as well as biologic end point to determine the historical disease control rate for patients with fully resected disease.
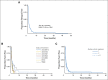
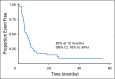
Results: In each included trial, the drugs tested were determined to be inactive on the basis of radiographic response rates. The EFS for 96 patients with osteosarcoma and measurable disease was 12% at 4 months (95% CI, 6% to 19%). There was no significant difference in EFS across trials according to number of prior treatment regimens or patient age, sex, and ethnicity. The 12-month EFS for the 42 evaluable patients enrolled in AOST0221 was 20% (95% CI, 10% to 34%).
Conclusion: The EFS was uniformly poor for children with recurrent/refractory osteosarcoma in these single-arm phase II trials. We have now constructed baseline EFS outcomes that can be used as a comparison for future phase II trials for recurrent osteosarcoma.
© 2016 by American Society of Clinical Oncology.
Conflict of interest statement
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Figures
References
-
- Link MP, Goorin AM, Miser AW, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986;314:1600–1606. - PubMed
-
- Meyers PA, Schwartz CL, Krailo M, et al. Osteosarcoma: A randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol. 2005;23:2004–2011. - PubMed
-
- Meyers PA, Schwartz CL, Krailo MD, et al. Osteosarcoma: The addition of muramyl tripeptide to chemotherapy improves overall survival—A report from the Children’s Oncology Group. J Clin Oncol. 2008;26:633–638. - PubMed
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. - PubMed
-
- Nesbit ME, Gehan EA, Burgert EO, et al: Multimodal therapy for the management of primary, nonmetastatic Ewing sarcoma of bone: A long-term follow-up of the first Intergroup Study. J Clin Oncol 8:1664-1674, 1990. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

