Elimination of Latently HIV-infected Cells from Antiretroviral Therapy-suppressed Subjects by Engineered Immune-mobilizing T-cell Receptors
- PMID: 27401039
- PMCID: PMC5154472
- DOI: 10.1038/mt.2016.114
Elimination of Latently HIV-infected Cells from Antiretroviral Therapy-suppressed Subjects by Engineered Immune-mobilizing T-cell Receptors
Abstract
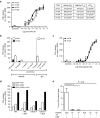
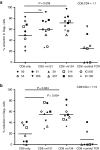
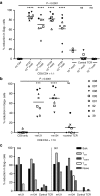
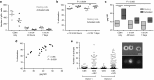
Persistence of human immunodeficiency virus (HIV) in a latent state in long-lived CD4+ T-cells is a major barrier to eradication. Latency-reversing agents that induce direct or immune-mediated cell death upon reactivation of HIV are a possible solution. However, clearance of reactivated cells may require immunotherapeutic agents that are fine-tuned to detect viral antigens when expressed at low levels. We tested the antiviral efficacy of immune-mobilizing monoclonal T-cell receptors against viruses (ImmTAVs), bispecific molecules that redirect CD8+ T-cells to kill HIV-infected CD4+ T-cells. T-cell receptors specific for an immunodominant Gag epitope, SL9, and its escape variants were engineered to achieve supraphysiological affinity and fused to a humanised CD3-specific single chain antibody fragment. Ex vivo polyclonal CD8+ T-cells were efficiently redirected by immune-mobilising monoclonal T-cell receptors against viruses to eliminate CD4+ T-cells from human histocompatibility leukocyte antigen (HLA)-A*0201-positive antiretroviral therapy-treated patients after reactivation of inducible HIV in vitro. The efficiency of infected cell elimination correlated with HIV Gag expression. Immune-mobilising monoclonal T-cell receptors against viruses have potential as a therapy to facilitate clearance of reactivated HIV reservoir cells.
Figures


Comment in
-
A New Agent in the Strategy to Cure AIDS.Mol Ther. 2016 Nov;24(11):1894-1896. doi: 10.1038/mt.2016.194. Mol Ther. 2016. PMID: 27916996 Free PMC article. No abstract available.
References
-
- Allers, K, Hütter, G, Hofmann, J, Loddenkemper, C, Rieger, K, Thiel, E et al. (2011). Evidence for the cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation. Blood 117: 2791–2799. - PubMed
-
- Autran, B, Descours, B and Bacchus, C (2013). Immune control of HIV-1 reservoirs. Curr Opin HIV AIDS 8: 204–210. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

