Optimization of Morpholino Antisense Oligonucleotides Targeting the Intronic Repressor Element1 in Spinal Muscular Atrophy
- PMID: 27401142
- PMCID: PMC5113110
- DOI: 10.1038/mt.2016.145
Optimization of Morpholino Antisense Oligonucleotides Targeting the Intronic Repressor Element1 in Spinal Muscular Atrophy
Abstract
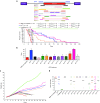
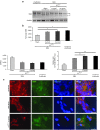
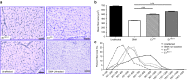
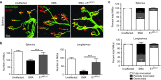
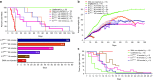
Loss of Survival Motor Neuron-1 (SMN1) causes Spinal Muscular Atrophy, a devastating neurodegenerative disease. SMN2 is a nearly identical copy gene; however SMN2 cannot prevent disease development in the absence of SMN1 since the majority of SMN2-derived transcripts are alternatively spliced, encoding a truncated, unstable protein lacking exon 7. Nevertheless, SMN2 retains the ability to produce low levels of functional protein. Previously we have described a splice-switching Morpholino antisense oligonucleotide (ASO) sequence that targets a potent intronic repressor, Element1 (E1), located upstream of SMN2 exon 7. In this study, we have assessed a novel panel of Morpholino ASOs with the goal of optimizing E1 ASO activity. Screening for efficacy in the SMNΔ7 mouse model, a single ASO variant was more active in vivo compared with the original E1(MO)-ASO. Sequence variant eleven (E1(MOv11)) consistently showed greater efficacy by increasing the lifespan of severe Spinal Muscular Atrophy mice after a single intracerebroventricular injection in the central nervous system, exhibited a strong dose-response across an order of magnitude, and demonstrated excellent target engagement by partially reversing the pathogenic SMN2 splicing event. We conclude that Morpholino modified ASOs are effective in modifying SMN2 splicing and have the potential for future Spinal Muscular Atrophy clinical applications.
Figures

References
-
- Monani, UR, Lorson, CL, Parsons, DW, Prior, TW, Androphy, EJ, Burghes, AH et al. (1999). A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum Mol Genet 8: 1177–1183. - PubMed
-
- Miyajima, H, Miyaso, H, Okumura, M, Kurisu, J and Imaizumi, K (2002). Identification of a cis-acting element for the regulation of SMN exon 7 splicing. J Biol Chem 277: 23271–23277. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

