Endothelial Nitric Oxide Synthase Traffic Inducer (NOSTRIN) is a Negative Regulator of Disease Aggressiveness in Pancreatic Cancer
- PMID: 27401251
- PMCID: PMC5161709
- DOI: 10.1158/1078-0432.CCR-16-0511
Endothelial Nitric Oxide Synthase Traffic Inducer (NOSTRIN) is a Negative Regulator of Disease Aggressiveness in Pancreatic Cancer
Abstract
Purpose: Pancreatic ductal adenocarcinoma (PDAC) is refractory to available treatments. Delineating critical pathways, responsible for disease aggressiveness and therapeutic resistance, may identify effective therapeutic targets. We aimed to identify key pathways contributing to disease aggressiveness by comparing gene expression profiles of tumors from early-stage PDAC cases with extremely poor survival (≤7 months) and those surviving 2 years or more following surgical resection.
Experimental design: Gene expression profiling was performed in tumors in a test cohort of PDAC (N = 50), which included short (≤7 months, N = 11) and long surviving (≥2 years, N = 14) patients, using affymetrix GeneChip Human 1.0 ST array. Key genes associated with disease aggressiveness were identified, using Cox regression, Kaplan-Meier, and pathway analyses with validations in independent cohorts for mechanistic and functional analyses.
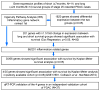
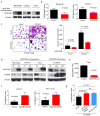
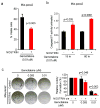
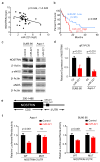
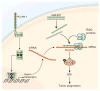
Results: Gene expression profiling identified 1,820 differentially expressed genes between short and long survival groups with inflammatory gene network ranking first. Lower expression of endothelial nitric oxide synthase traffic inducer (NOSTRIN) was associated with worst survival indicating its potential inhibitory role in disease progression. NOSTRIN overexpression suppressed migration and invasion of pancreatic cancer cells and enhanced sensitivity to chemotherapeutic drug gemcitabine. NOSTRIN inhibited production of nitric oxide (NO) by suppressing the activation of endothelial nitric oxide synthase (eNOS). Furthermore, miR-221, bound to the 3'UTR of NOSTRIN and suppressed its expression, and an increased miR-221 expression associated with poor survival in PDAC.
Conclusions: Our findings showed that NOSTRIN is a potential negative regulator of disease aggressiveness, which may be targeted for designing improved treatment strategy in PDAC. Clin Cancer Res; 22(24); 5992-6001. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
None
Figures

Similar articles
-
Nitric-oxide synthase trafficking inducer is a pleiotropic regulator of endothelial cell function and signaling.J Biol Chem. 2017 Apr 21;292(16):6600-6620. doi: 10.1074/jbc.M116.742627. Epub 2017 Feb 24. J Biol Chem. 2017. PMID: 28235804 Free PMC article.
-
Changes in human umbilical vein endothelial cells induced by endothelial nitric oxide synthase traffic inducer.J Huazhong Univ Sci Technolog Med Sci. 2013 Apr;33(2):272-276. doi: 10.1007/s11596-013-1110-2. Epub 2013 Apr 17. J Huazhong Univ Sci Technolog Med Sci. 2013. PMID: 23592143
-
DPEP1 inhibits tumor cell invasiveness, enhances chemosensitivity and predicts clinical outcome in pancreatic ductal adenocarcinoma.PLoS One. 2012;7(2):e31507. doi: 10.1371/journal.pone.0031507. Epub 2012 Feb 20. PLoS One. 2012. PMID: 22363658 Free PMC article.
-
NO• and Pancreatic Cancer: A Complex Interaction with Therapeutic Potential.Antioxid Redox Signal. 2017 Jun 10;26(17):1000-1008. doi: 10.1089/ars.2016.6809. Epub 2016 Sep 22. Antioxid Redox Signal. 2017. PMID: 27510096 Free PMC article. Review.
-
MiR-125a regulates chemo-sensitivity to gemcitabine in human pancreatic cancer cells through targeting A20.Acta Biochim Biophys Sin (Shanghai). 2016 Feb;48(2):202-8. doi: 10.1093/abbs/gmv129. Epub 2016 Jan 11. Acta Biochim Biophys Sin (Shanghai). 2016. PMID: 26758190
Cited by
-
Extensive protein S-nitrosylation associated with human pancreatic ductal adenocarcinoma pathogenesis.Cell Death Dis. 2019 Dec 4;10(12):914. doi: 10.1038/s41419-019-2144-6. Cell Death Dis. 2019. PMID: 31801946 Free PMC article.
-
PCDH1 promotes progression of pancreatic ductal adenocarcinoma via activation of NF-κB signalling by interacting with KPNB1.Cell Death Dis. 2022 Jul 21;13(7):633. doi: 10.1038/s41419-022-05087-y. Cell Death Dis. 2022. PMID: 35864095 Free PMC article.
-
Downregulation of CYB5D2 is associated with breast cancer progression.Sci Rep. 2019 Apr 29;9(1):6624. doi: 10.1038/s41598-019-43006-y. Sci Rep. 2019. PMID: 31036830 Free PMC article.
-
Biomarker significance of plasma and tumor miR-21, miR-221, and miR-106a in osteosarcoma.Oncotarget. 2017 May 27;8(57):96738-96752. doi: 10.18632/oncotarget.18236. eCollection 2017 Nov 14. Oncotarget. 2017. PMID: 29228567 Free PMC article.
-
Nitric-oxide synthase trafficking inducer is a pleiotropic regulator of endothelial cell function and signaling.J Biol Chem. 2017 Apr 21;292(16):6600-6620. doi: 10.1074/jbc.M116.742627. Epub 2017 Feb 24. J Biol Chem. 2017. PMID: 28235804 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer Statistics. CA Cancer J Clin. 2016;66:7–30. - PubMed
-
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

