SmartPill® as an objective parameter for determination of severity and duration of postoperative ileus: study protocol of a prospective, two-arm, open-label trial (the PIDuSA study)
- PMID: 27401360
- PMCID: PMC4947765
- DOI: 10.1136/bmjopen-2015-011014
SmartPill® as an objective parameter for determination of severity and duration of postoperative ileus: study protocol of a prospective, two-arm, open-label trial (the PIDuSA study)
Abstract
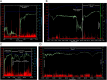
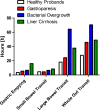
Introduction: Postoperative ileus (POI) is a frequent complication after abdominal surgery (AS). Until today, neither a prophylaxis nor an evidence-based therapy exists. This originates from the absence of objective parameters evaluating the severity and duration of POI resulting in clinical trials of modest quality. The SmartPill(®), a capsule which frequently measures pH value, temperature and intraluminal pressure after swallowing, offers an elegant option for analysing gastrointestinal (GI) transit times and smooth muscle activity in vivo. As the use in patients in the first months after AS is not covered by the marketing authorisation, we aim to investigate the safety and feasibility of the SmartPill(®) immediately after surgery. Additionally, we analyse the influence of prokinetics and laxatives as well as standardised physiotherapy on postoperative bowel contractility, as scientific evidence of its effects is still lacking.
Methods and analysis: The PIDuSA study is a prospective, single-centre, two-arm, open-label trial. The SmartPill(®) will be applied to 55 patients undergoing AS having a high risk for POI and 10 patients undergoing extra-abdominal surgery rarely developing POI. The primary objective is the safety of the SmartPill(®) in patients after surgery on the basis of adverse device effects/serious adverse device effects (ADE/SADE). The sample size suggests that events with a probability of 3% could be seen with a certainty of 80% for at least once in the sample. Secondary objective is the analysis of postoperative intestinal activity in the GI tract in both groups. Furthermore, clinical signs of bowel motility disorders will be correlated to the data measured by the SmartPill(®) to evaluate its significance as an objective parameter for assessing POI severity. Additionally, effects of prokinetics, laxatives and physiotherapy on postoperative peristaltic activity recorded by the SmartPill(®) will be analysed.
Ethics and dissemination: The protocol was approved by the federal authority (94.1.05-5660-8976) and the local ethics committee (092/14-MPG). Findings will be disseminated through publications and conference presentations.
Trial registration number: NCT02329912; Pre-results.
Keywords: SmartPill®; physiotherapy; postoperative ileus; prokinetic substances; safety; wireless motility capsule.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures


References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
