Population Pharmacokinetics of Fluconazole in Premature Infants with Birth Weights Less than 750 Grams
- PMID: 27401564
- PMCID: PMC4997840
- DOI: 10.1128/AAC.00963-16
Population Pharmacokinetics of Fluconazole in Premature Infants with Birth Weights Less than 750 Grams
Abstract
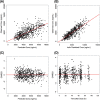
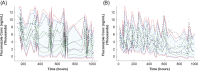
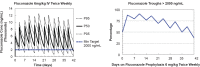
Fluconazole is an effective agent for prophylaxis of invasive candidiasis in premature infants. The objective of this study was to characterize the population pharmacokinetics (PK) and dosing requirements of fluconazole in infants with birth weights of <750 g. As part of a randomized clinical trial, infants born at <750 g birth weight received intravenous (i.v.) or oral fluconazole at 6 mg/kg of body weight twice weekly. Fluconazole plasma concentrations from samples obtained by either scheduled or scavenged sampling were measured using a liquid chromatography-tandem mass spectrometry assay. Population PK analysis was conducted using NONMEM 7.2. Population PK parameters were allometrically scaled by body weight. Covariates were evaluated by univariable screening followed by multivariable assessment. Fluconazole exposures were simulated in premature infants using the final PK model. A population PK model was developed from 141 infants using 604 plasma samples. Plasma fluconazole PK were best described by a one-compartment model with first-order elimination. Only serum creatinine was an independent predictor for clearance in the final model. The typical population parameter estimate for oral bioavailability in the final model was 99.5%. Scavenged samples did not bias the parameter estimates and were as informative as scheduled samples. Simulations indicated that the study dose maintained fluconazole troughs of >2,000 ng/ml in 80% of simulated infants at week 1 and 59% at week 4 of treatment. Developmental changes in fluconazole clearance are best predicted by serum creatinine in this population. A twice-weekly dose of 6 mg/kg achieves appropriate levels for prevention of invasive candidiasis in extremely premature infants.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
Population pharmacokinetics of fluconazole in young infants.Antimicrob Agents Chemother. 2008 Nov;52(11):4043-9. doi: 10.1128/AAC.00569-08. Epub 2008 Sep 22. Antimicrob Agents Chemother. 2008. PMID: 18809946 Free PMC article.
-
Population pharmacokinetics of fluconazole for prevention or treatment of invasive candidiasis in Chinese young infants.Naunyn Schmiedebergs Arch Pharmacol. 2024 Nov;397(11):8853-8862. doi: 10.1007/s00210-024-03184-7. Epub 2024 Jun 8. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38850301
-
Fluconazole Population Pharmacokinetics after Fosfluconazole Administration and Dosing Optimization in Extremely Low-Birth-Weight Infants.Microbiol Spectr. 2022 Apr 27;10(2):e0195221. doi: 10.1128/spectrum.01952-21. Epub 2022 Mar 10. Microbiol Spectr. 2022. PMID: 35266811 Free PMC article.
-
Fluconazole pharmacokinetics and safety in premature infants.Curr Med Chem. 2012;19(27):4617-20. doi: 10.2174/092986712803306367. Curr Med Chem. 2012. PMID: 22876898 Free PMC article. Review.
-
"Getting to Zero": preventing invasive Candida infections and eliminating infection-related mortality and morbidity in extremely preterm infants.Early Hum Dev. 2012 May;88 Suppl 2:S45-9. doi: 10.1016/S0378-3782(12)70014-2. Early Hum Dev. 2012. PMID: 22633513 Review.
Cited by
-
Preterm Physiologically Based Pharmacokinetic Model. Part II: Applications of the Model to Predict Drug Pharmacokinetics in the Preterm Population.Clin Pharmacokinet. 2020 Apr;59(4):501-518. doi: 10.1007/s40262-019-00827-4. Clin Pharmacokinet. 2020. PMID: 31587145 Clinical Trial.
-
Challenges in conducting paediatric trials with off-patent drugs.Contemp Clin Trials Commun. 2021 Jun 21;23:100783. doi: 10.1016/j.conctc.2021.100783. eCollection 2021 Sep. Contemp Clin Trials Commun. 2021. PMID: 34258467 Free PMC article.
-
Pharmacokinetics of antifungal drugs: practical implications for optimized treatment of patients.Infection. 2017 Dec;45(6):737-779. doi: 10.1007/s15010-017-1042-z. Epub 2017 Jul 12. Infection. 2017. PMID: 28702763 Free PMC article. Review.
-
A meta-analysis of fluconazole for the prevention of invasive fungal infection in preterm infants.Am J Transl Res. 2021 Feb 15;13(2):434-447. eCollection 2021. Am J Transl Res. 2021. PMID: 33594302 Free PMC article. Review.
-
Choosing the Right Antifungal Agent in ICU Patients.Adv Ther. 2019 Dec;36(12):3308-3320. doi: 10.1007/s12325-019-01115-0. Epub 2019 Oct 15. Adv Ther. 2019. PMID: 31617055 Free PMC article.
References
-
- Benjamin DK Jr, Stoll BJ, Fanaroff AA, McDonald SA, Oh W, Higgins RD, Duara S, Poole K, Laptook A, Goldberg R; National Institute of Child Health and Human Development Neonatal Research Network. 2006. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics 117:84–92. doi:10.1542/peds.2004-2292. - DOI - PubMed
-
- Manzoni P, Stolfi I, Pugni L, Decembrino L, Magnani C, Vetrano G, Tridapalli E, Corona G, Giovannozzi C, Farina D, Arisio R, Merletti F, Maule M, Mosca F, Pedicino R, Stronati M, Mostert M, Gomirato G; Italian Task Force for the Study and Prevention of Neonatal Fungal Infections; Italian Society of Neonatology. 2007. A multicenter, randomized trial of prophylactic fluconazole in preterm neonates. N Engl J Med 356:2483–2495. doi:10.1056/NEJMoa065733. - DOI - PubMed
-
- Benjamin DK Jr, Hudak ML, Duara S, Randolph DA, Bidegain M, Mundakel GT, Natarajan G, Burchfield DJ, White RD, Shattuck KE, Neu N, Bendel CM, Kim MR, Finer NN, Stewart DL, Arrieta AC, Wade KC, Kaufman DA, Manzoni P, Prather KO, Testoni D, Berezny KY, Smith PB; Fluconazole Prophylaxis Study Team. 2014. Effect of fluconazole prophylaxis on candidiasis and mortality in premature infants: a randomized clinical trial. JAMA 311:1742–1749. doi:10.1001/jama.2014.2624. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHSN267200700051C/HD/NICHD NIH HHS/United States
- K24 HD058735/HD/NICHD NIH HHS/United States
- R01 HD081044/HD/NICHD NIH HHS/United States
- K23 HD068497/HD/NICHD NIH HHS/United States
- HHSN275201000003I/HD/NICHD NIH HHS/United States
- UL1 TR001117/TR/NCATS NIH HHS/United States
- HHSN275201000003C/AA/NIAAA NIH HHS/United States
- HHSN272201500006C/AI/NIAID NIH HHS/United States
- R01 FD003519/FD/FDA HHS/United States
- R01 HD057956/HD/NICHD NIH HHS/United States
- R34 HL124038/HL/NHLBI NIH HHS/United States
- R18 FD005292/FD/FDA HHS/United States
- R21 HD080606/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

