Impact of Time to Appropriate Therapy on Mortality in Patients with Vancomycin-Intermediate Staphylococcus aureus Infection
- PMID: 27401565
- PMCID: PMC4997841
- DOI: 10.1128/AAC.00925-16
Impact of Time to Appropriate Therapy on Mortality in Patients with Vancomycin-Intermediate Staphylococcus aureus Infection
Abstract
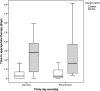
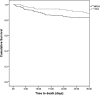
Despite the increasing incidence of vancomycin-intermediate Staphylococcus aureus (VISA) infections, few studies have examined the impact of delay in receipt of appropriate antimicrobial therapy on outcomes in VISA patients. We examined the effects of timing of appropriate antimicrobial therapy in a cohort of patients with sterile-site methicillin-resistant S. aureus (MRSA) and VISA infections. In this single-center, retrospective cohort study, we identified all patients with MRSA or VISA sterile-site infections from June 2009 to February 2015. Clinical outcomes were compared according to MRSA/VISA classification, demographics, comorbidities, and antimicrobial treatment. Thirty-day all-cause mortality was modeled with Kaplan-Meier curves. Multivariate logistic regression analysis (MVLRA) was used to determine odds ratios for mortality. We identified 354 patients with MRSA (n = 267) or VISA (n = 87) sterile-site infection. Fifty-five patients (15.5%) were nonsurvivors. Factors associated with mortality in MVLRA included pneumonia, unknown source of infection, acute physiology and chronic health evaluation (APACHE) II score, solid-organ malignancy, and admission from skilled care facilities. Time to appropriate antimicrobial therapy was not significantly associated with outcome. Presence of a VISA infection compared to that of a non-VISA S. aureus infection did not result in excess mortality. Linezolid use was a risk for mortality in patients with APACHE II scores of ≥14. Our results suggest that empirical vancomycin use in patients with VISA infections does not result in excess mortality. Future studies should (i) include larger numbers of patients with VISA infections to confirm the findings presented here and (ii) determine the optimal antibiotic therapy for critically ill patients with MRSA and VISA infections.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R. 2013. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41:580–637. doi: 10.1097/CCM.0b013e31827e83af. - DOI - PubMed
-
- Soriano A, Marco F, Martinez JA, Pisos E, Almela M, Dimova VP, Alamo D, Ortega M, Lopez J, Mensa J. 2008. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis 46:193–200. doi: 10.1086/524667. - DOI - PubMed
-
- Casapao AM, Leonard SN, Davis SL, Lodise TP, Patel N, Goff DA, Laplante KL, Potoski BA, Rybak MJ. 24 June 2013. Clinical outcomes in patients with heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA) bloodstream infection. Antimicrob Agents Chemother doi: 10.1128/aac.00380-13. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

