Role of ABC and Solute Carrier Transporters in the Placental Transport of Lamivudine
- PMID: 27401571
- PMCID: PMC4997848
- DOI: 10.1128/AAC.00648-16
Role of ABC and Solute Carrier Transporters in the Placental Transport of Lamivudine
Abstract
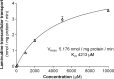
Lamivudine is one of the antiretroviral drugs of choice for the prevention of mother-to-child transmission (MTCT) in HIV-positive women. In this study, we investigated the relevance of drug efflux transporters P-glycoprotein (P-gp) (MDR1 [ABCB1]), BCRP (ABCG2), MRP2 (ABCC2), and MATE1 (SLC47A1) for the transmembrane transport and transplacental transfer of lamivudine. We employed in vitro accumulation and transport experiments on MDCK cells overexpressing drug efflux transporters, in situ-perfused rat term placenta, and vesicular uptake in microvillous plasma membrane (MVM) vesicles isolated from human term placenta. MATE1 significantly accelerated lamivudine transport in MATE1-expressing MDCK cells, whereas no transporter-driven efflux of lamivudine was observed in MDCK-MDR1, MDCK-MRP2, and MDCK-BCRP monolayers. MATE1-mediated efflux of lamivudine appeared to be a low-affinity process (apparent Km of 4.21 mM and Vmax of 5.18 nmol/mg protein/min in MDCK-MATE1 cells). Consistent with in vitro transport studies, the transplacental clearance of lamivudine was not affected by P-gp, BCRP, or MRP2. However, lamivudine transfer across dually perfused rat placenta and the uptake of lamivudine into human placental MVM vesicles revealed pH dependency, indicating possible involvement of MATE1 in the fetal-to-maternal efflux of the drug. To conclude, placental transport of lamivudine does not seem to be affected by P-gp, MRP2, or BCRP, but a pH-dependent mechanism mediates transport of lamivudine in the fetal-to-maternal direction. We suggest that MATE1 might be, at least partly, responsible for this transport.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures

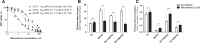


Similar articles
-
Interactions of tenofovir and tenofovir disoproxil fumarate with drug efflux transporters ABCB1, ABCG2, and ABCC2; role in transport across the placenta.AIDS. 2014 Jan 2;28(1):9-17. doi: 10.1097/QAD.0000000000000112. AIDS. 2014. PMID: 24413260
-
Efavirenz reduces renal excretion of lamivudine in rats by inhibiting organic cation transporters (OCT, Oct) and multidrug and toxin extrusion proteins (MATE, Mate).PLoS One. 2018 Aug 16;13(8):e0202706. doi: 10.1371/journal.pone.0202706. eCollection 2018. PLoS One. 2018. PMID: 30114293 Free PMC article.
-
Interactions between Maraviroc and the ABCB1, ABCG2, and ABCC2 Transporters: An Important Role in Transplacental Pharmacokinetics.Drug Metab Dispos. 2019 Sep;47(9):954-960. doi: 10.1124/dmd.119.087684. Epub 2019 Jul 2. Drug Metab Dispos. 2019. PMID: 31266750
-
[Expression and regulation of drug transporters in placenta].Yao Xue Xue Bao. 2016 Jun;51(6):879-85. Yao Xue Xue Bao. 2016. PMID: 29878741 Review. Chinese.
-
Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense.Toxicol Appl Pharmacol. 2005 May 1;204(3):216-37. doi: 10.1016/j.taap.2004.10.012. Toxicol Appl Pharmacol. 2005. PMID: 15845415 Review.
Cited by
-
An update on placental drug transport and its relevance to fetal drug exposure.Med Rev (2021). 2022 Nov 21;2(5):501-511. doi: 10.1515/mr-2022-0025. eCollection 2022 Oct. Med Rev (2021). 2022. PMID: 37724167 Free PMC article. Review.
-
MDR1 and BCRP Transporter-Mediated Drug-Drug Interaction between Rilpivirine and Abacavir and Effect on Intestinal Absorption.Antimicrob Agents Chemother. 2017 Aug 24;61(9):e00837-17. doi: 10.1128/AAC.00837-17. Print 2017 Sep. Antimicrob Agents Chemother. 2017. PMID: 28696229 Free PMC article.
-
S-(4-Nitrobenzyl)-6-thioinosine (NBMPR) is Not a Selective Inhibitor of Equilibrative Nucleoside Transporters but Also Blocks Efflux Activity of Breast Cancer Resistance Protein.Pharm Res. 2020 Feb 21;37(3):58. doi: 10.1007/s11095-020-2782-5. Pharm Res. 2020. PMID: 32086630
-
Multiple Drug Transporters Contribute to the Placental Transfer of Emtricitabine.Antimicrob Agents Chemother. 2019 Jul 25;63(8):e00199-19. doi: 10.1128/AAC.00199-19. Print 2019 Aug. Antimicrob Agents Chemother. 2019. PMID: 31160284 Free PMC article.
-
An update on expression and function of P-gp/ABCB1 and BCRP/ABCG2 in the placenta and fetus.Expert Opin Drug Metab Toxicol. 2018 Aug;14(8):817-829. doi: 10.1080/17425255.2018.1499726. Epub 2018 Aug 3. Expert Opin Drug Metab Toxicol. 2018. PMID: 30010462 Free PMC article. Review.
References
-
- UNAIDS. 2015. Fact sheet 2014. Global statistics 2014; UNAIDS, Geneva, Switzerland: http://www.unaids.org/en/media/unaids/contentassets/documents/factsheet/....
-
- WHO. 2013. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. World Health Organization, Geneva, Switzerland. - PubMed
-
- Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. 2015. Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf Accessed 26 May 2016.
-
- Wong F, Pai R, Van Schalkwyk J, Yoshida EM. 2014. Hepatitis B in pregnancy: a concise review of neonatal vertical transmission and antiviral prophylaxis. Ann Hepatol 13:187–195. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

