Dynamic polarization vision in mantis shrimps
- PMID: 27401817
- PMCID: PMC4945877
- DOI: 10.1038/ncomms12140
Dynamic polarization vision in mantis shrimps
Abstract
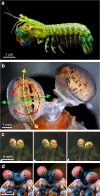
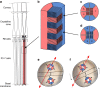
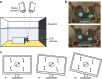
Gaze stabilization is an almost ubiquitous animal behaviour, one that is required to see the world clearly and without blur. Stomatopods, however, only fix their eyes on scenes or objects of interest occasionally. Almost uniquely among animals they explore their visual environment with a series pitch, yaw and torsional (roll) rotations of their eyes, where each eye may also move largely independently of the other. In this work, we demonstrate that the torsional rotations are used to actively enhance their ability to see the polarization of light. Both Gonodactylus smithii and Odontodactylus scyllarus rotate their eyes to align particular photoreceptors relative to the angle of polarization of a linearly polarized visual stimulus, thereby maximizing the polarization contrast between an object of interest and its background. This is the first documented example of any animal displaying dynamic polarization vision, in which the polarization information is actively maximized through rotational eye movements.
Figures


References
-
- Land M. F. Motion and vision: why animals move their eyes. J. Comp. Physiol. A 185, 341–352 (1999). - PubMed
-
- Land M. F. Head movement of flies during visually guided flight. Nature 243, 299–300 (1973).
-
- Troje N. F. & Frost B. J. Head-bobbing in pigeons: how stable is the hold phase? J. Exp. Biol. 203, 935–940 (2000). - PubMed
-
- Zeil J. & Hemmi J. M. The visual ecology of fiddler crabs. J. Comp. Physiol. A 192, 1–25 (2006). - PubMed
-
- Layne J. E., Wicklein M., Dodge F. A. & Barlow R. B. Prediction of maximum allowable retinal slip speed in the fiddler crab Uca pugilator. Biol. Bull 193, 202–203 (1997). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

