Structure and Pharmacologic Modulation of Inhibitory Glycine Receptors
- PMID: 27401877
- PMCID: PMC4998662
- DOI: 10.1124/mol.116.105726
Structure and Pharmacologic Modulation of Inhibitory Glycine Receptors
Abstract
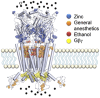
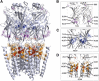
Glycine receptors (GlyR) are inhibitory Cys-loop ion channels that contribute to the control of excitability along the central nervous system (CNS). GlyR are found in the spinal cord and brain stem, and more recently they were reported in higher regions of the CNS such as the hippocampus and nucleus accumbens. GlyR are involved in motor coordination, respiratory rhythms, pain transmission, and sensory processing, and they are targets for relevant physiologic and pharmacologic modulators. Several studies with protein crystallography and cryoelectron microscopy have shed light on the residues and mechanisms associated with the activation, blockade, and regulation of pentameric Cys-loop ion channels at the atomic level. Initial studies conducted on the extracellular domain of acetylcholine receptors, ion channels from prokaryote homologs-Erwinia chrysanthemi ligand-gated ion channel (ELIC), Gloeobacter violaceus ligand-gated ion channel (GLIC)-and crystallized eukaryotic receptors made it possible to define the overall structure and topology of the Cys-loop receptors. For example, the determination of pentameric GlyR structures bound to glycine and strychnine have contributed to visualizing the structural changes implicated in the transition between the open and closed states of the Cys-loop receptors. In this review, we summarize how the new information obtained in functional, mutagenesis, and structural studies have contributed to a better understanding of the function and regulation of GlyR.
Copyright © 2016 by The American Society for Pharmacology and Experimental Therapeutics.
Figures
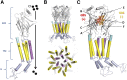
Similar articles
-
Ethanol Modulation is Quantitatively Determined by the Transmembrane Domain of Human α1 Glycine Receptors.Alcohol Clin Exp Res. 2015 Jun;39(6):962-8. doi: 10.1111/acer.12735. Epub 2015 May 14. Alcohol Clin Exp Res. 2015. PMID: 25973519 Free PMC article.
-
Multisite binding of a general anesthetic to the prokaryotic pentameric Erwinia chrysanthemi ligand-gated ion channel (ELIC).J Biol Chem. 2013 Mar 22;288(12):8355-8364. doi: 10.1074/jbc.M112.424507. Epub 2013 Jan 30. J Biol Chem. 2013. PMID: 23364792 Free PMC article.
-
Microsecond simulations indicate that ethanol binds between subunits and could stabilize an open-state model of a glycine receptor.Biophys J. 2011 Apr 6;100(7):1642-50. doi: 10.1016/j.bpj.2011.02.032. Biophys J. 2011. PMID: 21463577 Free PMC article.
-
Molecular pharmacology of the glycine receptor chloride channel.Curr Pharm Des. 2007;13(23):2350-67. doi: 10.2174/138161207781368693. Curr Pharm Des. 2007. PMID: 17692006 Review.
-
Inhibitory glycine receptors: an update.J Biol Chem. 2012 Nov 23;287(48):40216-23. doi: 10.1074/jbc.R112.408229. Epub 2012 Oct 4. J Biol Chem. 2012. PMID: 23038260 Free PMC article. Review.
Cited by
-
Aging in nucleus accumbens and its impact on alcohol use disorders.Alcohol. 2023 Mar;107:73-90. doi: 10.1016/j.alcohol.2022.08.004. Epub 2022 Sep 7. Alcohol. 2023. PMID: 36087859 Free PMC article. Review.
-
Interactions Involving Glycine and Other Amino Acid Neurotransmitters: Focus on Transporter-Mediated Regulation of Release and Glycine-Glutamate Crosstalk.Biomedicines. 2024 Jul 8;12(7):1518. doi: 10.3390/biomedicines12071518. Biomedicines. 2024. PMID: 39062091 Free PMC article. Review.
-
Interactions between Glycine and Glutamate through Activation of Their Transporters in Hippocampal Nerve Terminals.Biomedicines. 2023 Nov 27;11(12):3152. doi: 10.3390/biomedicines11123152. Biomedicines. 2023. PMID: 38137373 Free PMC article.
-
The cellular mechanisms associated with the anesthetic and neuroprotective properties of xenon: a systematic review of the preclinical literature.Front Neurosci. 2023 Jul 14;17:1225191. doi: 10.3389/fnins.2023.1225191. eCollection 2023. Front Neurosci. 2023. PMID: 37521706 Free PMC article.
-
Presence of ethanol-sensitive glycine receptors in medium spiny neurons in the mouse nucleus accumbens.J Physiol. 2017 Aug 1;595(15):5285-5300. doi: 10.1113/JP273767. Epub 2017 Jun 23. J Physiol. 2017. PMID: 28524260 Free PMC article.
References
-
- Absalom NL, Lewis TM, Kaplan W, Pierce KD, Schofield PR. (2003) Role of charged residues in coupling ligand binding and channel activation in the extracellular domain of the glycine receptor. J Biol Chem 278:50151–50157. - PubMed
-
- Aguayo LG. (1990) Ethanol potentiates the GABAA-activated Cl− current in mouse hippocampal and cortical neurons. Eur J Pharmacol 187:127–130. - PubMed
-
- Aguayo LG, Pancetti FC. (1994) Ethanol modulation of the γ-aminobutyric acidA- and glycine-activated Cl− current in cultured mouse neurons. J Pharmacol Exp Ther 270:61–69. - PubMed
-
- Aguayo LG, Tapia JC, Pancetti FC. (1996) Potentiation of the glycine-activated Cl− current by ethanol in cultured mouse spinal neurons. J Pharmacol Exp Ther 279:1116–1122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

