Clinical trial network for the promotion of clinical research for rare diseases in Japan: muscular dystrophy clinical trial network
- PMID: 27401940
- PMCID: PMC4939632
- DOI: 10.1186/s12913-016-1477-4
Clinical trial network for the promotion of clinical research for rare diseases in Japan: muscular dystrophy clinical trial network
Abstract
Background: Duchenne muscular dystrophy (DMD) is the most commonly inherited neuromuscular disease. Therapeutic agents for the treatment of rare disease, namely "orphan drugs", have recently drawn the attention of researchers and pharmaceutical companies. To ensure the successful conduction of clinical trials to evaluate novel treatments for patients with rare diseases, an appropriate infrastructure is needed. One of the effective solutions for the lack of infrastructure is to establish a network of rare diseases.
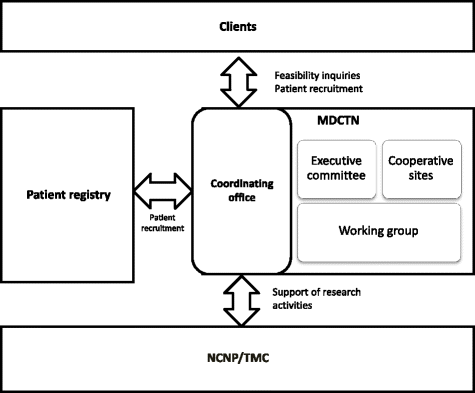
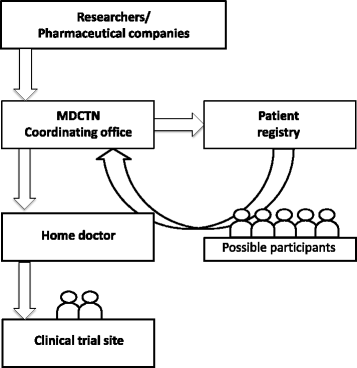
Methods: To accomplish the conduction of clinical trials in Japan, the Muscular dystrophy clinical trial network (MDCTN) was established by the clinical research group for muscular dystrophy, including the National Center of Neurology and Psychiatry, as well as national and university hospitals, all which have a long-standing history of research cooperation.
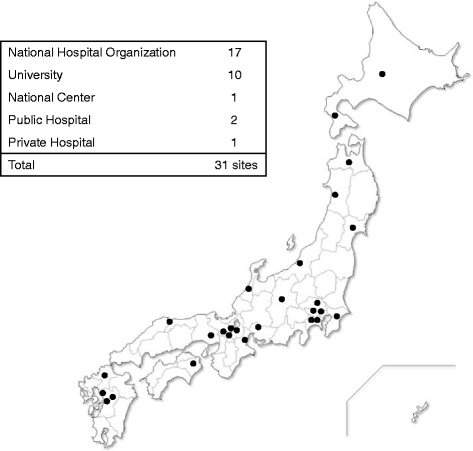
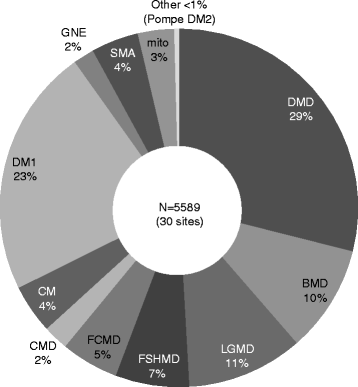
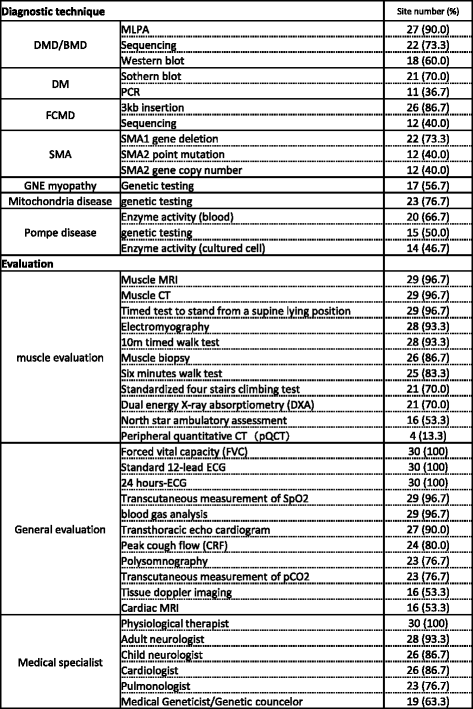
Results: Thirty-one medical institutions (17 national hospital organizations, 10 university hospitals, 1 national center, 2 public hospitals, and 1 private hospital) belong to this network and collaborate to facilitate clinical trials. The Care and Treatment Site Registry (CTSR) calculates and reports the proportion of patients with neuromuscular diseases in the cooperating sites. In total, there are 5,589 patients with neuromuscular diseases in Japan and the proportion of patients with each disease is as follows: DMD, 29 %; myotonic dystrophy type 1, 23 %; limb girdle muscular dystrophy, 11 %; Becker muscular dystrophy, 10 %. We work jointly to share updated health care information and standardized evaluations of clinical outcomes as well. The collaboration with the patient registry (CTSR), allows the MDCTN to recruit DMD participants with specific mutations and conditions, in a remarkably short period of time.
Conclusion: Counting with a network that operates at a national level is important to address the corresponding national issues. Thus, our network will be able to contribute with international research activity, which can lead to an improvement of neuromuscular disease treatment in Japan.
Keywords: Clinical trial network; Muscular dystrophy; Muscular dystrophy clinical trial network (MDCTN); Neuromuscular diseases; Orphan drugs; Rare diseases; Registry of Muscular Dystrophy (Remudy).
Figures
Similar articles
-
[Infrastructure for the clinical research of muscular dystrophies: remudy and MDCTN].Rinsho Shinkeigaku. 2014;54(12):1069-70. doi: 10.5692/clinicalneurol.54.1069. Rinsho Shinkeigaku. 2014. PMID: 25519964 Review. Japanese.
-
[Registry of muscular dystrophy (Remudy). Construction of the patient self-report registry and collaboration with overseas network].Rinsho Shinkeigaku. 2011 Nov;51(11):901-2. doi: 10.5692/clinicalneurol.51.901. Rinsho Shinkeigaku. 2011. PMID: 22277410 Japanese.
-
[Infrastructure for new drug development to treat muscular dystrophy: current status of patient registration (remudy)].Brain Nerve. 2011 Nov;63(11):1279-84. Brain Nerve. 2011. PMID: 22068481 Review. Japanese.
-
[Remudy].Brain Nerve. 2014 Nov;66(11):1396-402. doi: 10.11477/mf.1416200045. Brain Nerve. 2014. PMID: 25407075 Review. Japanese.
-
[Establishment of an evaluation method for muscular dystrophy and a patient registration system for clinical trials].Rinsho Shinkeigaku. 2009 Nov;49(11):863-6. doi: 10.5692/clinicalneurol.49.863. Rinsho Shinkeigaku. 2009. PMID: 20030232 Review. Japanese.
Cited by
-
From promising molecules to orphan drugs: Early clinical drug development.Intractable Rare Dis Res. 2017 Feb;6(1):29-34. doi: 10.5582/irdr.2016.01094. Intractable Rare Dis Res. 2017. PMID: 28357178 Free PMC article. Review.
-
STRATEGIES TO ENHANCE RECRUITMENT METHODS IN PHANTOM LIMB PAIN CLINICAL TRIALS.Int J Clin Trials. 2017 Apr-Jun;4(2):72-79. doi: 10.18203/2349-3259.ijct20171917. Int J Clin Trials. 2017. PMID: 29683138 Free PMC article.
References
-
- Ministry of Health LaW. Overview of Orphan Drug/Medical Device Designation System: Ministry of Health, Labour and Welfare; [cited 2014 27 Febrary]. http://www.mhlw.go.jp/english/policy/health-medical/pharmaceuticals/orph....
-
- CINRG. [cited 2014 27 Febrary]. http://www.cinrgresearch.org/.
-
- TREAT-NMD. TREAT-NMD [cited 2014 27 Febrary]. http://www.treat-nmd.eu/.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

